Abstract
Gastric cancer is the most common cancer in Korea and has overall survival rate of around 50%. Gastric cancer detected in early stage can be cured by endoscopic resection or less invasive surgical treatment and the subsequent prognosis is excellent. National cancer screening program for gastric cancer has been available for several years. The evaluation for efficacy of our screening strategy is strongly needed in terms of mortality reduction and cost-effectiveness. Accurate diagnosis and staging evaluation is important for proper management and prediction of a patient's prognosis. It is recommended to understand the advantages and limitations of currently available guidelines and diagnostic modalities. The 7th edition of gastric cancer staging system from AJCC may have significant effect on our knowledge and patient management.
REFERENCES
1. Ministry for Health, Welfare and Family Affairs. Annual Report of the Korea Central Cancer Registry. 2008.
2. Ministry for Health, Welfare and Family Affairs & National Cancer Center. Cancer Facts and Figures. 2008.
3. Choi KS, Kwak MS, Lee HY, Jun JK, Hahm MI, Park EC. Screening for gastric cancer in Korea: population-based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol Biomarkers Prev. 2009; 18:1390–1398.

5. Kong SH, Park DJ, Lee HJ, et al. Clinicopathologic features of asymptomatic gastric adenocarcinoma patients in Korea. Jpn J Clin Oncol. 2004; 34:1–7.

6. Nam SY, Choi IJ, Park KW, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009; 21:855–860.

7. Gates TJ. Screening for cancer: evaluating the evidence. Am Fam Physician. 2001; 63:513–522.
8. Miyamoto A, Kuriyama S, Nishino Y, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med. 2007; 44:12–19.

9. Oshima A, Hirata N, Ubukata T, Umeda K, Fujimoto I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer. 1986; 38:829–833.

10. Fukao A, Tsubono Y, Tsuji I, HIsamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995; 60:45–48.

11. Abe Y, Mitsushima T, Nagatani K, Ikuma H, Minamihara Y. Epidemiological evaluation of the protective effect for dying of stomach cancer by screening programme for stomach cancer with applying a method of case-control study–a study of a efficient screening programme for stomach cancer. Jpn J Gastroenterol. 1995; 92:836–845.
12. Hisamichi S, Sugawara N. Mass screening for gastric cancer by X-ray examination. Jpn J Clin Oncol. 1984; 14:211–223.
13. Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006; 118:2315–2321.

14. Oshima A, Hanai A, Fujimoto I. Evaluation of a mass screening program for stomach cancer. Natl Cancer Inst Monogr. 1979; 53:181–186.
15. Inaba S, Hirayama H, Nagata C, et al. Evaluation of a screening program on reduction of gastric cancer mortality in Japan: preliminary results from a cohort study. Prev Med. 1999; 29:102–106.
16. Mizoue T, Yoshimura T, Tokui N, et al. Japan Collaborative Cohort Study Group. Prospective study of screening for stomach cancer in Japan. Int J Cancer. 2003; 106:103–107.
17. Hosokawa O, Miyanaga T, Kaizaki Y, et al. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008; 43:1112–1115.

18. Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006; 12:4873–4874.
19. Mori Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer. 2001; 4:132–136.

20. Riecken B, Pfeiffer R, Ma JL, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002; 34:22–28.

21. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992; 52:6735–6740.
22. Kim JH, Kim HY, Kim NY, et al. Korea H. pylori Study Group, South Korea. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001; 16:969–975.
23. Wong BC, Lam SK, Wong WM, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004; 291:187–194.
24. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English edition. Gastric Cancer. 1998; 1:10–24.
25. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(suppl 6):S3–S43.
26. Sobin LH, Wittekind C. UICC: TNM classification of malignant tumours. 6th ed.New York: Wiley-Liss;2002. 65-68.
27. Japenese Research Society for Gastric Cancer. Japenese classification of gastric carcinoma; general rules for the gastric cancer study. 1st ed.Tokyo: Kanehara;1995. p. 1–104.
28. Fleming ID, Cooper JS, Henson DE, et al. AJCC cancer staging manual. 5th ed.Philadelphia: Lippincott-Raven;1997.
29. Wittekind C. News in the TNM classification. In WHO-CC for gastric cancer. 12th international seminar. 6th general assembly. Seoul, Korea. 1996.
30. Park DJ, Kong SH, Lee HJ, et al. Subclassification of pT2 gastric adenocarcinoma according to depth of invasion (pT2a vs pT2b) and lymph node status (pN). Surgery. 2007; 141:757–763. Epub 2007 May 4.

31. Lu Y, Liu C, Zhang R, et al. Prognostic significance of subclassification of pT2 gastric cancer: a retrospective study of 847 patients. Surg Oncol. 2008; 17:317–322.

32. Kim JP, Hur YS, Yang HK. Lymph node metastasis as a significant prognostic factor in early gastric cancer: analysis of 1,136 early gastric cancers. Ann Surg Oncol. 1995; 2:308–313.

33. Shen JY, Kim S, Cheong JH, et al. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer. 2007; 110:745–751.

34. Marchet A, Mocellin S, Ambrosi A, et al; Italian Research Group for Gastric Cancer (IRGGC). The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007; 245:543–552.
35. Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009; 16:1868–1874.

36. Lee JM, Jung SE. Imaging diagnosis of gastric cancer: CT scan. J Korean Radiol Soc. 2002; 46:511–519.

37. D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000; 10:1877–1885.
38. Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004; 230:465–471.

39. Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998; 22:288–294.

40. Hundt W, Braunschweig R, Reiser M. Assessment of gastric cancer: value of breathhold technique and two-phase spiral CT. Eur Radiol. 1999; 9:68–72.

41. Hur J, Park MS, Lee JH, et al. Diagnostic accuracy of multidetector row computed tomography in T- and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006; 30:372–377.

42. Kim HJ, Kim AY, Oh ST, et al. Gastric cancer staging at multidetector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005; 236:879–885.

43. Xi WD, Zhao C, Ren GS. Endoscopic ultrasonography in preoperative staging of gastric cancer: determination of tumor invasion depth, nodal involvement and surgical resectability. World J Gastroenterol. 2003; 9:254–257.

44. Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc. 2000; 14:951–954.
45. Bhandari S, Shim CS, Kim JH, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004; 59:619–626.

46. Ganpathi IS, So JB, Ho KY. Endoscopic ultrasonography for gastric cancer: does it influence treatment? Surg Endosc. 2006; 20:559–562.
47. Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol. 2007; 14:1853–1859.

48. Chung IK. Debate on ESD: EUS, PET/CT. Korean J Gastrointest Endosc. 2009; 38(suppl 1):S177–S183.
49. Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG PET for detection of gastric cancer. Gastric Cancer. 2006; 9:192–196.
50. Kim SK, Kang KW, Lee JS, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155.

51. Hamilton SR, Aaltonen LA. WHO classification of tumours. Pathology and genetics. Tumours of the digestive system. Lyon: IARC Press;2000.
52. Kim WH, Park CK, Kim YB, et al; The Gastrointestinal Pathology Study Group of the Korean Society of Pathologists. A standardized pathology report for gastric cancer. Korean J Pathol. 2005; 39:106–113.
Fig. 1.
Subtypes of advanced gastric cancer (Type 1-4). Type I, fungating type; Type II, ulcerofungating type; Type III, ulcer-oinfiltrative type; Type IV, infiltrative type.
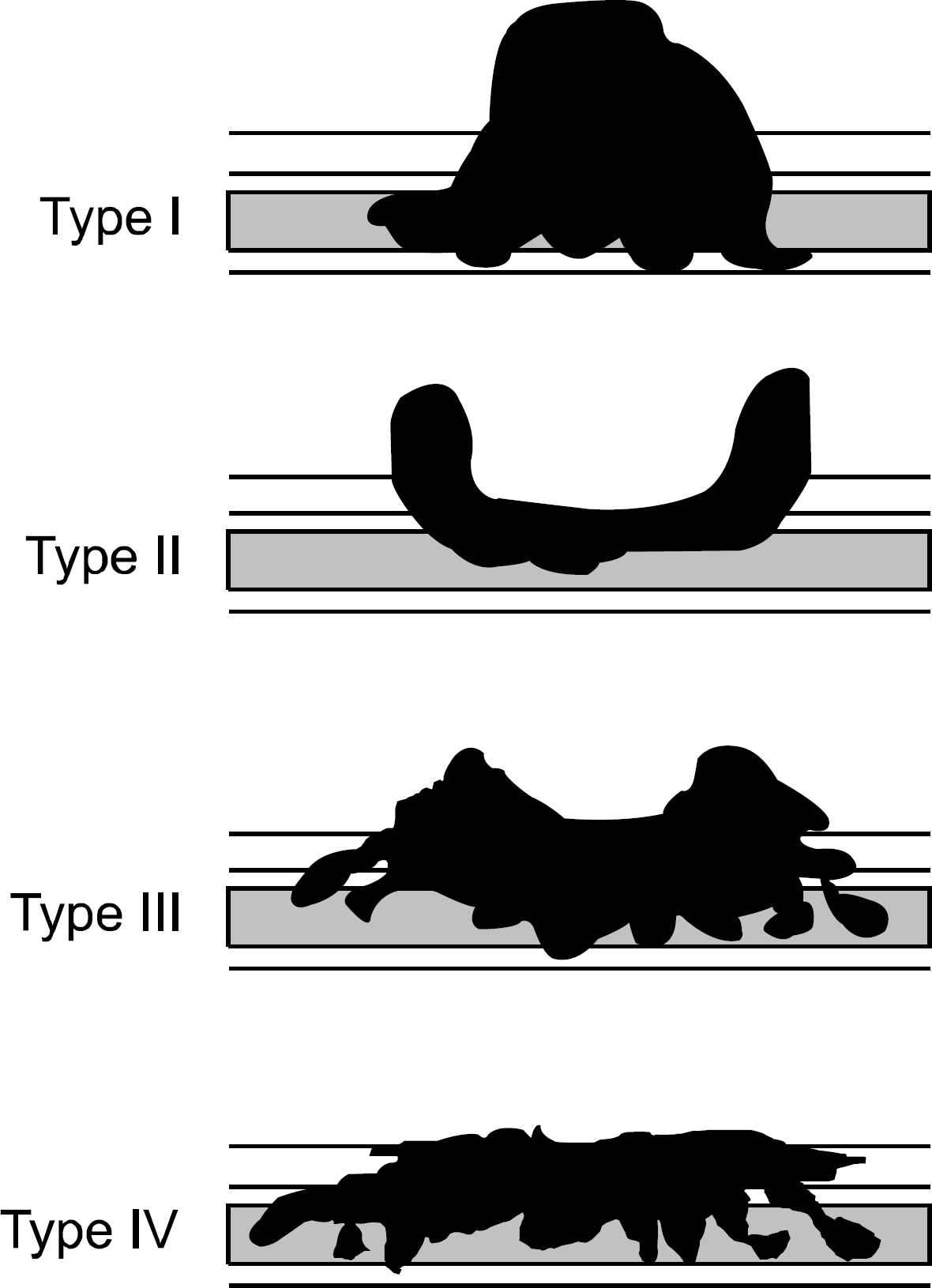
Fig. 2.
Subtypes of early gastric cancer (Type 0). Type 0-I, protruded type; Type 0-IIa, superficial elevated type; Type 0-IIb, flat type; Type 0-IIc superficial depressed type; Type 0-III, excavated type.
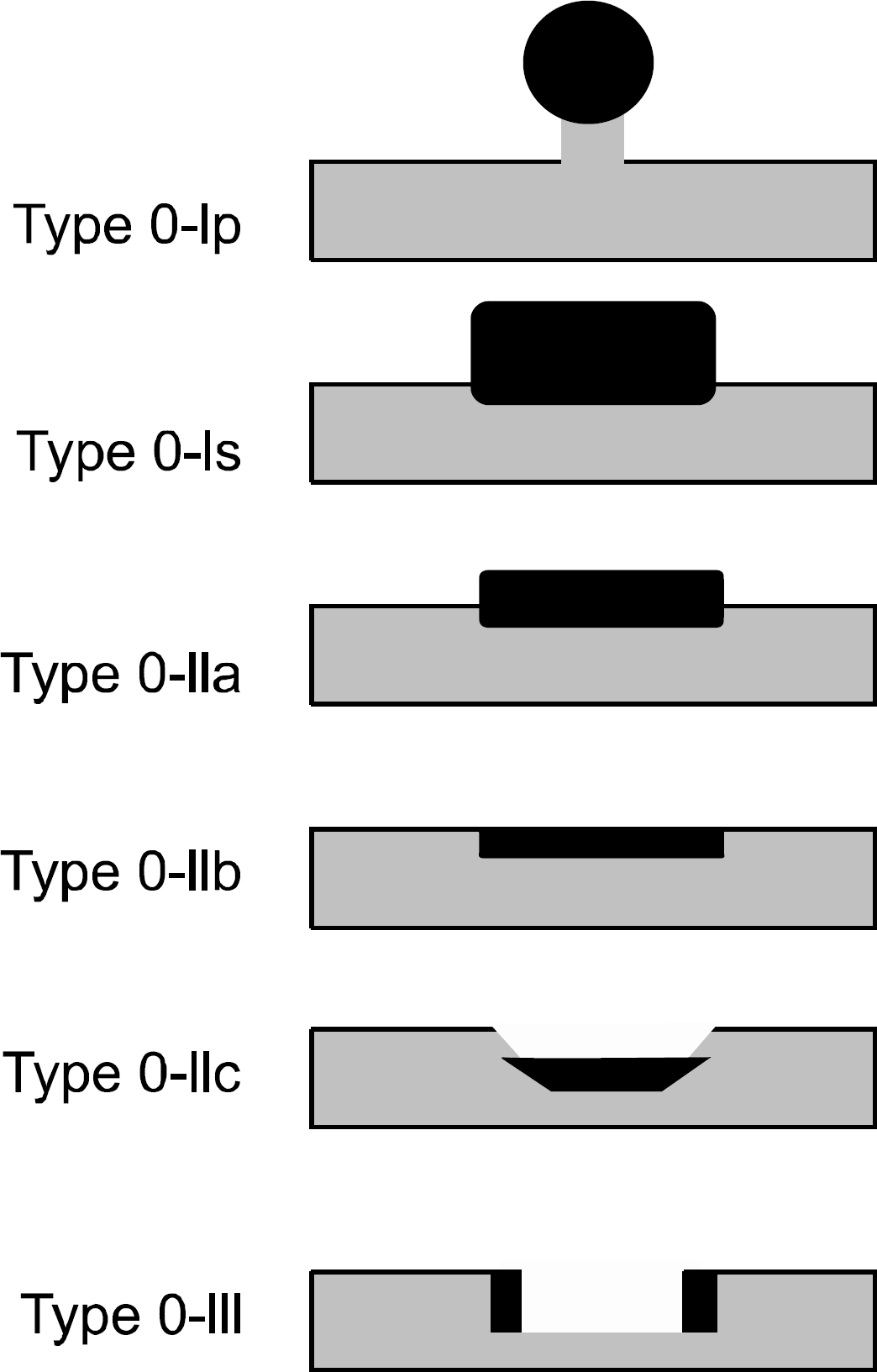
Fig. 3.
Schematic view of tumor location. Stomach is longitudinally divided into three equal parts, and cross-sectionally divided into four parts.
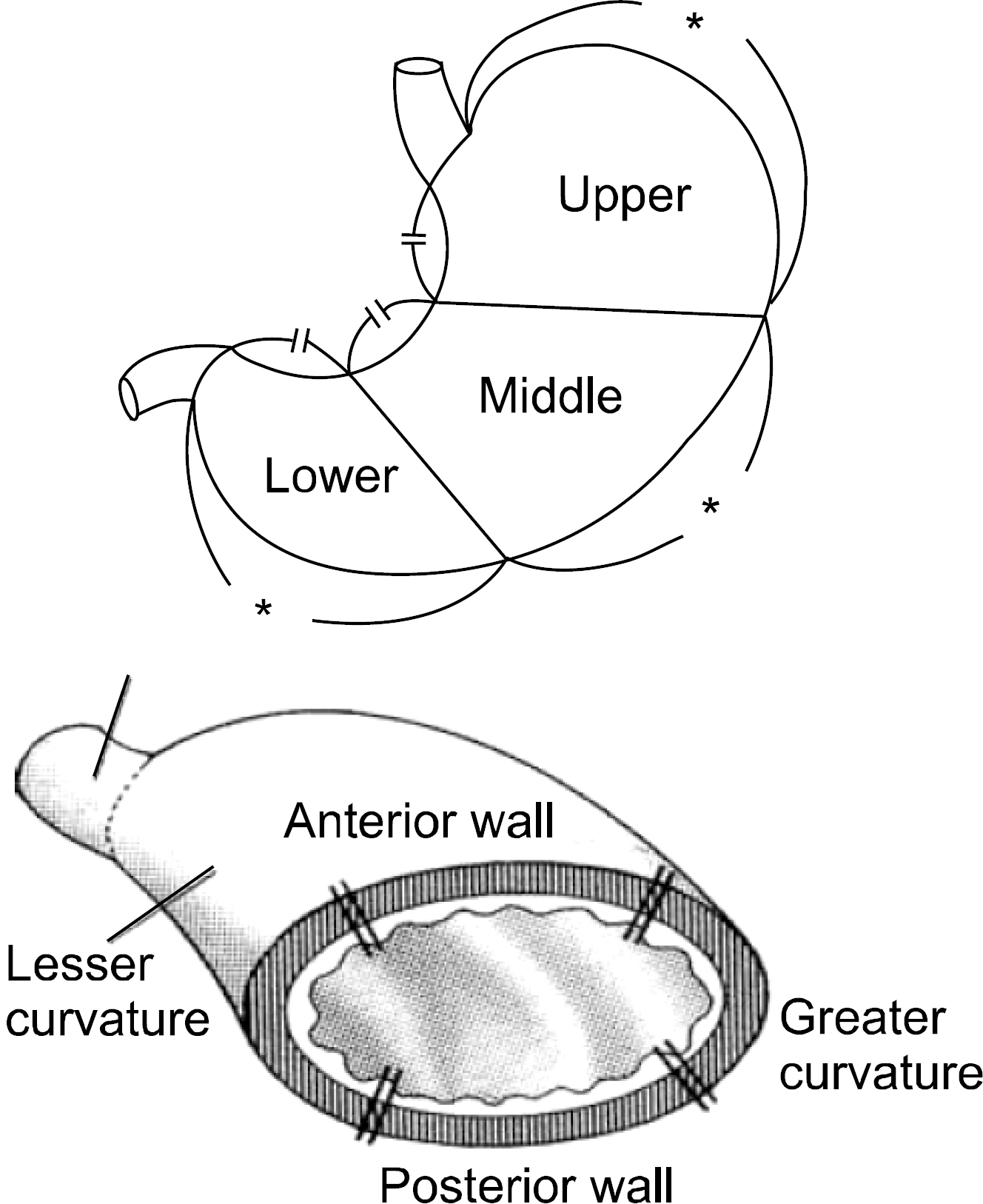
Table 1.
Summary of Case-control Studies and Cohort Studies Evaluated Screening Effect on Mortality of Gastric Cancer Performed in Japan by Photofluorography
| Reference | Population | Follow-up | Age | No of subject | Results |
|---|---|---|---|---|---|
| Case-Control study | Odds ratio | ||||
| Oshima et al.9 | Osaka | 40+ | 91 case | M: 0.60 (0.34-1.05) | |
| 261 control | F: 0.38 (0.19-0.79) | ||||
| Fukao et al.10 | Miyagi | 50+ | 198 case | 0.41 (0.28-0.61) | |
| 577 control | |||||
| Abe et al.11 | Chiba | 30+ | 820 case | M: 0.37 (0.34-1.05) | |
| 2,413 control | F: 0.46 (0.26-0.80) | ||||
| Cohort study | Relative risks | ||||
| Oshima et al.14 | Osaka | 1967-1975 (6 yr) | All age | 32,789 | 0.91∗ |
| Hisamichi and | Miyagi | 1960-1977 (18 yr) | 40-69 | 7,008 | 61.9 (p<0.005)† |
| Sugawara12 | 28.1 (p<0.01)‡ | ||||
| Inaba et al.15 | Gifu | 1992-1995 (40 month) | 40< | 24,134 | M: 0.72 (0.31-1.66) |
| F: 1.46 (0.43-4.90) | |||||
| Mizoue et al.16 | Inaba (JACC) | 1988-1997 (8 yr) | 50-69 | 87,312 | M: 0.65 (0.45-0.95) |
| F: 0.75 (0.42-1.34) | |||||
| Lee et al.13 | JPHC | 1990-2003 (13 yr) | 40-59 | 42,150 | 0.52 (0.36-0.74) |
| Miyamoto et al.8 | Miyagi | 1990-2001 (11 yr) | 41,394 | 0.54 (0.38-0.77) |
Table 2.
Gastic Cancer Staging System (AJCC 6th ed)
Table 3.
Gastic Cancer Stage Grouping (AJCC 6th ed)
Table 4.
A Standardized Pathology Reporting Format for Gastric Cancer52




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download