Abstract
Background/Aims
The aim of this study was to elucidate the antiviral efficacy of lamivudine (LMV)-adefovir (ADV) combination therapy in chronic hepatitis B patients who showed resistance to LMV and ADV consecutively.
Methods
A retrospective review was performed in eighteen patients with chronic hepatitis B who developed virologic breakthroughs during LMV-ADV sequential monotherapy and treated with LMV-ADV combination therapy.
Results
The median duration of follow up was 17 months (range, 6-27) after the start of LMV-ADV combination therapy. Mean HBV DNA level in log10 IU/mL was 6.08±0.95, 4.05±1.66, 3.17±1.58, 3.18±2.16, and 2.35±1.52 at 0, 3, 6, 12, and 24 months, respectively. Sixteen patients (88.9%) showed HBV DNA reduction below detection limit (<20,000 IU/mL). HBeAg seroconversion was observed in one patient (7.1%) after 8 months of combination therapy. Virologic breakthrough occurred in only one patient after 21 months of combination therapy. Viral rebound occurred in two patients at 12 months and 14 months of combination therapy. Normalization of serum ALT was achieved in twelve patients (66.7%). Primary non-response was observed in two cases (11.1%).
REFERENCES
1. Karayiannis P. Hepatitis B virus: old, new and future approaches to antiviral treatment. J Antimicrob Chemother. 2003; 51:761–785.

2. Lau DT, Khokhar MF, Doo E, et al. Longterm therapy of chronic hepatitis B with lamivudine. Hepatology. 2003; 32:828–834.

3. Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003; 348:808–816.
4. Yotsuyanagi H, Koike K. Drug resistance in antiviral treatment for infections with hepatitis B and C viruses. J Gastroenterol. 2007; 42:329–335.

5. Tim S, Scott B, Stetphen L. Rescue therapy for drug resistant hepatitis B: another argument for combination chemo-atherapy? Gastroenterology. 2004; 126:343–350.
6. Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48weeks of adefovir dipivoxil monotherapy. Hepatology. 2006; 43:1385–1391.
7. Yeon JE, Yoo W, Hong SP, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006; 55:1488–1495.

8. van Bömmel F, Zöllner B, Sarrazin C, et al. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006; 44:318–324.

9. Villet S, Pichoud C, Villeneuve JP, Trepo C, Zoulim F. Selection of a multipe drug-resistant hepatitis B virus strain in a liver-transplanted patient. Gastroenterology. 2006; 131:1253–1261.
10. Ratziu V, Thibault V, Benhamou Y, Polynard T. Successful rescue therapy with tenofovir in a patient with hepatic de-compensation and adefovir resistant HBV mutant. Comp Hepatol. 2006; 5:1.

12. Sasadeusz JJ, Locarnini SL, Macdonald G. Why do we not yet have combination chemotherapy for chronic hepatitis B? Med J Aust. 2007; 186:204–206.

13. Marceliin P, Asselah T. Resistance to adefovir: a new chal-lenge in the treatment of chronic hepatitis B. J Hepatol. 2005; 43:920–923.
14. Mutimer D. Adefovir-lamivudine combination therapy and hepatitis B viral kinetics. J Hepatol. 2005; 43:200–202.

15. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summury of a clinical research workshop. Hepatology. 2007; 45:1056–1075.
16. Aloman C, Wands JR. Resistance of HBV to Adefovir Dipivoxil: a case for combination antiviral therapy. Hepatology. 2003; 38:1584–1587.

17. Augus P, Vaughan R, Xiong S, et al. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003; 125:292–297.

18. Brunelle MN, Jacquard AC, Pichoud C, et al. Susceptibility to antivirals of a human HBV strain with mutations confer-ring resistance to both lamivudine and adefovir. Hepatology. 2005; 41:1391–1398.

19. Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006; 44:703–712.

20. 권소영, 최원혁, 연종은 등. 라미부딘-아데포비어 sequential monotherapy에 내성을 보인 만성 B형간염 환자에서 라미부딘 병합치료의 항바이러스 효과와 내성 변이 발현에 관한 연구. 대한간학회 춘계학술대회 초록집. 2006; 12(suppl 3):S81.
Fig. 1.
A flow chart illustrating the outcome of Lamivudine-Adefovir combination therapy in HBV infected patients with sequential resistance.∗ Chronic hepatitis B, fall of HBV DNA below 20,000 IU/mL and normal alanine aminotransferase levels after 3 months of entecavir therapy. † Compensated liver cirrhosis, 5 log10 IU/mL of HBV DNA and mildly elevated alanine aminotransferase levels but no compensation during LMV/ADV combination therapy for 18 months. ADV, adefovir; LMV, lamivudine.
Fig. 2.
Mean log changes in serum HBV DNA levels during Lamivudine-Adefovir combination therapy. HBV DNA declined rapidly at initial 3 months of combination therapy then slowly decreased throughout the observation period.
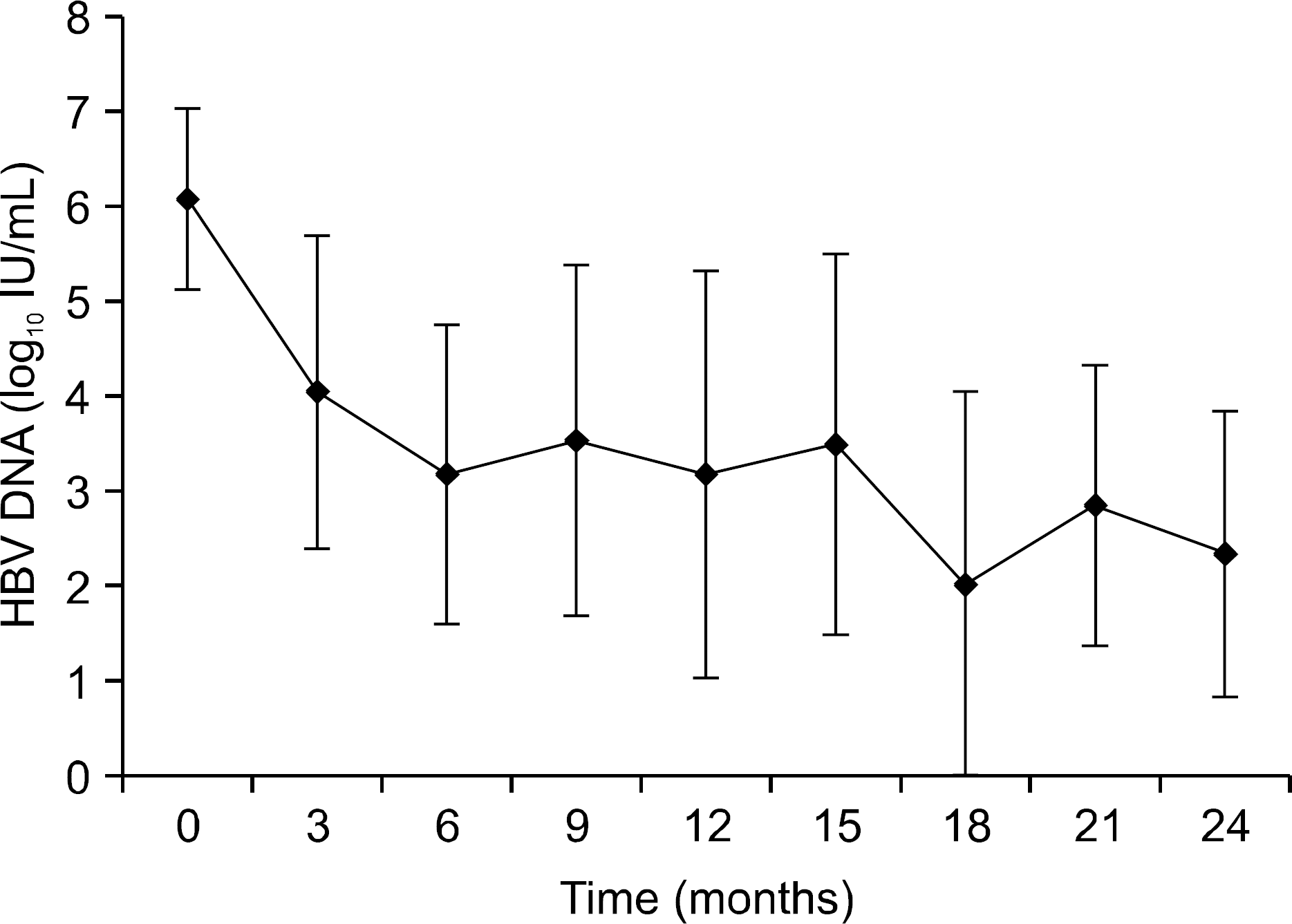
Fig. 3.
Mean ALT changes in serum HBV DNA levels during Lamivudine-Adefovir combination therapy. ALT declined rapidly at initial 3 months of treamemt and gradually declined throughout the observation period.
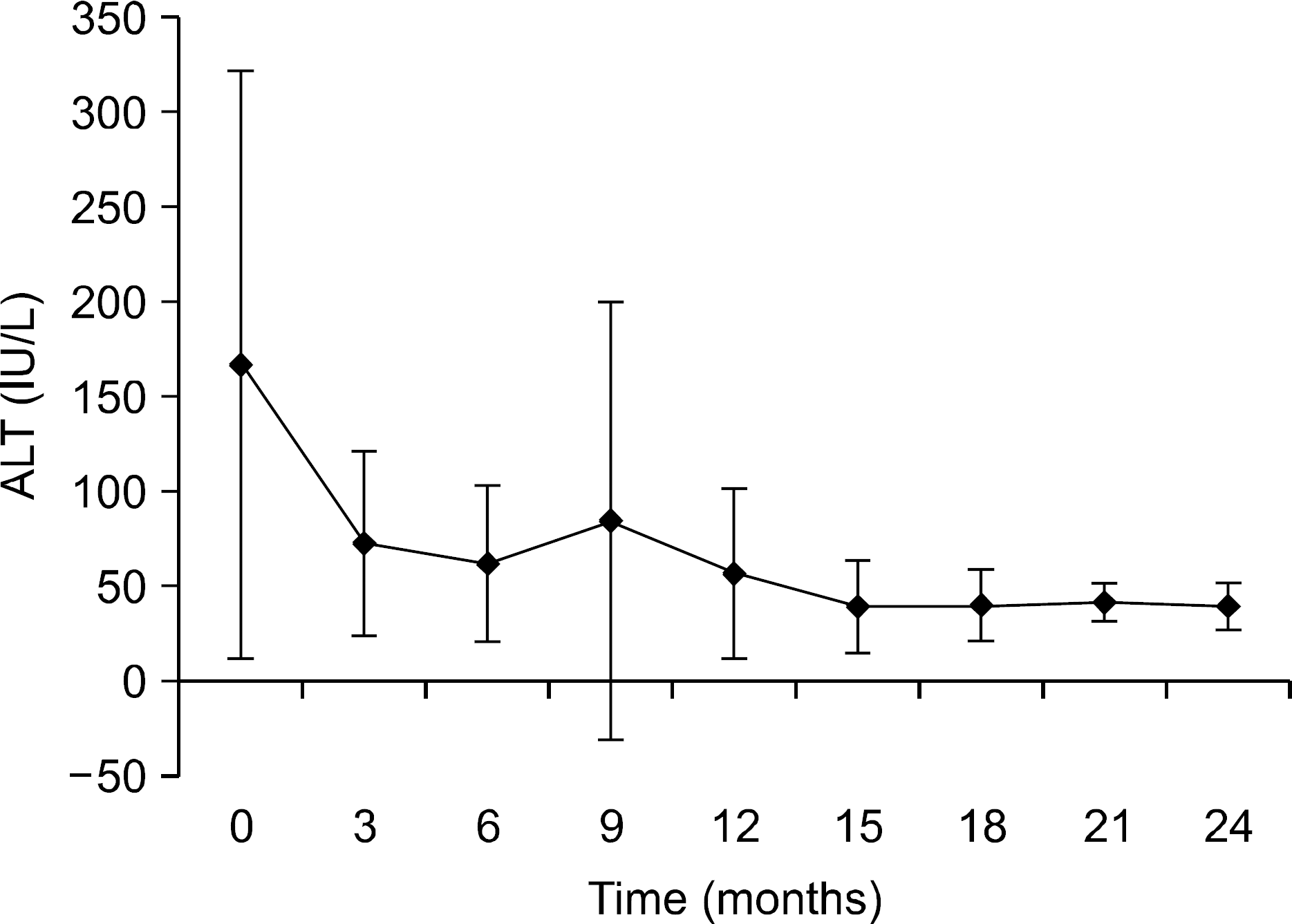
Table 1.
Baseline Characteristics of Patients with Lamivudine-Adefovir Combination Therapy
| Total (n=18) | |
|---|---|
| Age in years, median (range) | 52 (24-62) |
| Male:Female | 14:4 |
| Median duration of follow-up in month (range) | 17 (6-27) |
| Liver disease | |
| Chronic hepatitis B | 7 |
| Liver cirrhosis, compensated | 8 |
| Liver cirrhosis, decompensated | 3 |
| Prior ADV therapy in months, | 18 (6-26) |
| median (range) | |
| HBeAg (%) | |
| Positive | 14 (77.8) |
| Negative | 4 (22.2) |
| Log10 HBV DNA IU/mL, mean (± SD) | 6.09 (±0.95) |
| Serum ALT in IU/L, mean (± SD) | 167.78 (±154.65) |
| Genotypic resistance to LMV | 16∗ |
| rtM204I | 5 |
| rtM204I/rtL180M | 5 |
| rtM204V/rtL180M | 3 |
| rtM204I/rtM204V/rtL180M | 2 |
| Genotypic resistance to ADV | 6 |
| rtA181V | 3 |
| rtA181T | 1 |
| rtN236T | 1 |
| rtA181V/rtN236T | 1 |
Table 2.
Clinical Course of Lamivudine-Adefovir Combination Therapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download