Abstract
Figures and Tables
Fig. 1
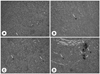
Table 2

1) Values are mean ± SE of 7 rats per group, 2) NS: not significantly different among groups, 3) Values with the different letters in the same column are significantly different at (p < 0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet
Table 3

1) Values are mean ± SE of 7 rats per group, 2) NS: not significantly different among groups, 3) Values with the different letters in the same column are significantly different at (p <0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet, GOT: glutamate oxalacetate transferase, GPT: glutamate pyruvate transferase, BUN: blood urea nitrogen, Cre: creatinine, UA: uric acid, ALP: alkaline phosphatase, PTH: parathyroid hormone
Table 4

1) Values are mean ± SE of 7 rats per group, 2) Values with the different letters in the same column are significantly different at (p < 0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet
*: Average values of femurs
†: Lumbar No.2-5
Table 5

1) Values are mean ± SE of 7 rats per group, 2) NS: not significantly different among groups, 3) Values with the different letters in the same column are significantly different at (p < 0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet
Table 6

1) Values are mean ± SE of 7 rats per group, 2) Values with the different letters in the same column are significantly different at (p< 0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet. ND: not detected
*: Average wet weight of kidneys
†: Left kidney
Table 7

1) Right kidney
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet, Mineralization grade (count): None(0), Mild (2-5), Moderate (6-8), Severe (11-31)
Table 8

1) Values are mean ± SE of 7 rats per group, 2) Values with the different letters in the same column are significantly different at (p< 0.05) by Duncan's multiple range test
Sham/NCa: Sham operation and normal Ca diet (0.5%), Ovx/NCa: ovariectomy operation and normal Ca diet (0.5%), Ovx/NCa-ox: ovariectomy operation and normal Ca (0.5%) with sodium oxalate (1%) diet, Ovx/LCa: ovariectomy operation and low Ca diet (0.1%), Ovx/LCa-ox: ovariectomy operation and low Ca (0.1%) with sodium oxalate (1%) diet




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download