Abstract
Purpose
This study aimed to investigate the potential of freeze-dried persimmon powder (Diospyros kaki Thumb.) to protect against dyslipidemia induced by a high-fat/cholesterol diet (HFD) in a rat model. Methods: Fifty Wistar rats were randomly divided into five groups: normal control (NC), high-fat/cholesterol control (HC), tannin in HFD (HT, 1% of diet), immature persimmon in HFD (HI, 7% of diet), and mature persimmon in HFD (HM, 7% of diet). Tannin was used as a positive control. Biochemical, molecular, and histopathological changes were observed in the blood and liver. Results: We confirmed that a high fat/cholesterol diet successfully induced dyslipidemia, which was characterized by significantly altered lipid profiles in the plasma and liver. However, oxidized low-density lipoprotein levels, histopathological damage in the liver, and hepatic triglyceride levels were significantly reduced in all HT, HI, and HM groups compared to those in the HF group. In contrast, plasma apolipoprotein B level was significantly reduced only in the HT and HM groups, whereas reduction of the LDL-C level was detected only in the HI group. Although HF-induced sterol regulatory element-binding protein (SREBP) gene expression was significantly reduced in all treated groups, downstream gene expression levels varied among the different groups; significant reduction of fatty acid synthase (FAS) and 3-hydroxy-3-methylglutaryl-CoA (HMGCR) gene expression was detected only in the HI group, whereas cholesterol 7α-hydroxylase (CYP7A1) gene expression was significantly elevated only in the HM group. Conclusion: Taken together, the data suggest that protection of LDL oxidation and hepatic lipogenesis might be, at least partly, attributed to tannin in persimmons. However, the identified mechanisms varied up to the maturation stage of persimmon. In the case of immature persimmon, modulation of FAS and HMGCR gene expression was prominent, whereas in the case of mature persimmon, modulation of CYP7A1 gene expression was prominent.
References
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
2. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2015: Korea National Health and Nutrition Examination Survey (KNHANES VI-3). Sejong: Korea Centers for Disease Control and Prevention;2016.
4. Al-Mohaissen MA, Ignaszewski MJ, Frohlich J, Ignaszewski AP. Statin-associated muscle adverse events: update for clinicians. Sultan Qaboos Univ Med J. 2016; 16(4):e406–e415.

5. Duell PB, Connor WE. Vitamin D deficiency is associated with myalgias in hyperlipidemic subjects taking statins. Circulation. 2008; 118(Suppl 18):S470.

6. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007; 25(10):1135–1151.

7. Bian LL, You SY, Park J, Yang SJ, Chung HJ. Characteristics of nutritional components in astringent persimmons according to growing region and cultivar. J Korean Soc Food Sci Nutr. 2015; 44(3):379–385.

8. Yokozawa T, Park CH, Noh JS, Roh SS. Role of oligomeric proanthocyanidins derived from an extract of persimmon fruits in the oxidative stress-related aging process. Molecules. 2014; 19(5):6707–6726.

9. Jang IC, Jo EK, Bae MS, Lee HJ, Jeon GI, Park E, Yuk HG, Ahn GH, Lee SH. Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J Med Plant Res. 2010; 4(2):155–160.
10. Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol (Tokyo). 2007; 53(3):287–292.

11. Lee JS, Lee MK, Ha TY, Bok SH, Park HM, Jeong KS, Woo MN, Do GM, Yeo JY, Choi MS. Supplementation of whole persimmon leaf improves lipid profiles and suppresses body weight gain in rats fed high-fat diet. Food Chem Toxicol. 2006; 44(11):1875–1883.

12. Matsumoto K, Yokoyama S, Gato N. Hypolipidemic effect of young persimmon fruit in C57BL/6.KOR-ApoEshl mice. Biosci Biotechnol Biochem. 2008; 72(10):2651–2659.
13. Gorinstein S, Bartnikowska E, Kulasek G, Zemser M, Trakhtenberg S. Dietary persimmon improves lipid metabolism in rats fed diets containing cholesterol. J Nutr. 1998; 128(11):2023–2027.

14. Matsumoto K, Yokoyama S, Gato N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother Res. 2010; 24(2):205–210.

15. Gorinstein S, Leontowicz H, Leontowicz M, Jesion I, Namiesnik J, Drzewiecki J, Park YS, Ham KS, Giordani E, Trakhtenberg S. Influence of two cultivars of persimmon on atherosclerosis indices in rats fed cholesterol-containing diets: Investigation in vitro and in vivo. Nutrition. 2011; 27(7–8):838–846.

16. Zou B, Ge ZZ, Zhang Y, Du J, Xu Z, Li CM. Persimmon tannin accounts for hypolipidemic effects of persimmon through activating of AMPK and suppressing NF-κB activation and inflammatory responses in high-fat diet rats. Food Funct. 2014; 5(7):1536–1546.

17. Zou B, Li C, Chen J, Dong X, Zhang Y, Du J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res Int. 2012; 48(2):970–977.

18. Matsumoto K, Kadowaki A, Ozaki N, Takenaka M, Ono H, Yokoyama S, Gato N. Bile acid-binding ability of kaki-tannin from young fruits of persimmon (Diospyros kaki) in vitro and in vivo. Phytother Res. 2011; 25(4):624–628.

19. Matsumoto K, Yokoyama S. Induction of uncoupling protein-1 and −3 in brown adipose tissue by kaki-tannin in type 2 diabetic NSY/Hos mice. Food Chem Toxicol. 2012; 50(2):184–190.

20. Gorinstein S, Kulasek GW, Bartnikowska E, Leontowicz M, Zemser M, Morawiec M, Trakhtenberg S. The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J Nutr Biochem. 1998; 9(4):223–227.

21. Gorinstein S, Kulasek GW, Bartnikowska E, Leontowicz M, Zemser M, Morawiec M, Trakhtenberg S. The effects of diets, supplemented with either whole persimmon or phenol-free persimmon, on rats fed cholesterol. Food Chem. 2000; 70(3):303–308.

22. Park YS, Leontowicz H, Leontowicz M, Namiesnik J, Jesion I, Gorinstein S. Nutraceutical value of persimmon (Diospyros kaki Thunb.) and its influence on some indices of atherosclerosis in an experiment on rats fed cholesterol-containing diet. Adv Hortic Sci. 2008; 22(4):250–254.
23. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226(1):497–509.

24. Seong JH, Han JP. The qualitative differences of persimmon tannin and the natural removal of astringency. Korean J Postharvest Sci Technol. 1999; 6(1):66–70.
25. Beninger CW, Hosfield GL. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J Agric Food Chem. 2003; 51(27):7879–7883.

26. Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009; 53(Suppl 2):S310–S329.

27. Gu HF, Li CM, Xu Y, Hu W, Chen M, Wan Q. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res Int. 2008; 41(2):208–217.

28. Gato N, Kadowaki A, Hashimoto N, Yokoyama S, Matsumoto K. Persimmon fruit tannin-rich fiber reduces cholesterol levels in humans. Ann Nutr Metab. 2013; 62(1):1–6.

29. Karaman S, Toker ÖS, Yüksel F, Çam M, Kayacier A, Dogan M. Physicochemical, bioactive, and sensory properties of persimmon-based ice cream: technique for order preference by similarity to ideal solution to determine optimum concentration. J Dairy Sci. 2014; 97(1):97–110.

30. Lee SH. Diagnosis and treatment of dyslipidemia. Korean J Med. 2008; 74(4):358–362.
31. Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998; 101(11):2331–2339.

32. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006; 86(2):465–514. 14707.

33. Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J, Harada K, Gotoda T, Sato R, Kimura S, Ishibashi S, Yamada N. Transcriptional activities of nuclear SREBP-1a, −1c, and −2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002; 43(8):1220–1235.

34. Willnow TE, Sheng Z, Ishibashi S, Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994; 264(5164):1471–1474.

35. Osono Y, Woollett LA, Herz J, Dietschy JM. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest. 1995; 95(3):1124–1132.

36. Temel RE, Brown JM. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol Sci. 2015; 36(7):440–451.

37. Venkateswaran A, Repa JJ, Lobaccaro JM, Bronson A, Mangels-dorf DJ, Edwards PA. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J Biol Chem. 2000; 275(19):14700–14707.
Fig. 1.
Effects of immature and mature persimmon on food intakes and body weight changes in rats fed a high-fat/cholesterol diet: (A) food intakes and (B) total body weight gain. NC; normal control, HF; high-fat/cholesterol control, HT; high-fat/cholesterol diet with 1% tannin, HI; high-fat/cholesterol diet with 7% immature persimmon, HM; high-fat/cholesterol diet with 7% mature persimmon. Values are expressed as means ± SE (n = 10 for each group). Means with different letters at each panel are significantly different from each other at p < 0.05 by Duncan's multiple range test.
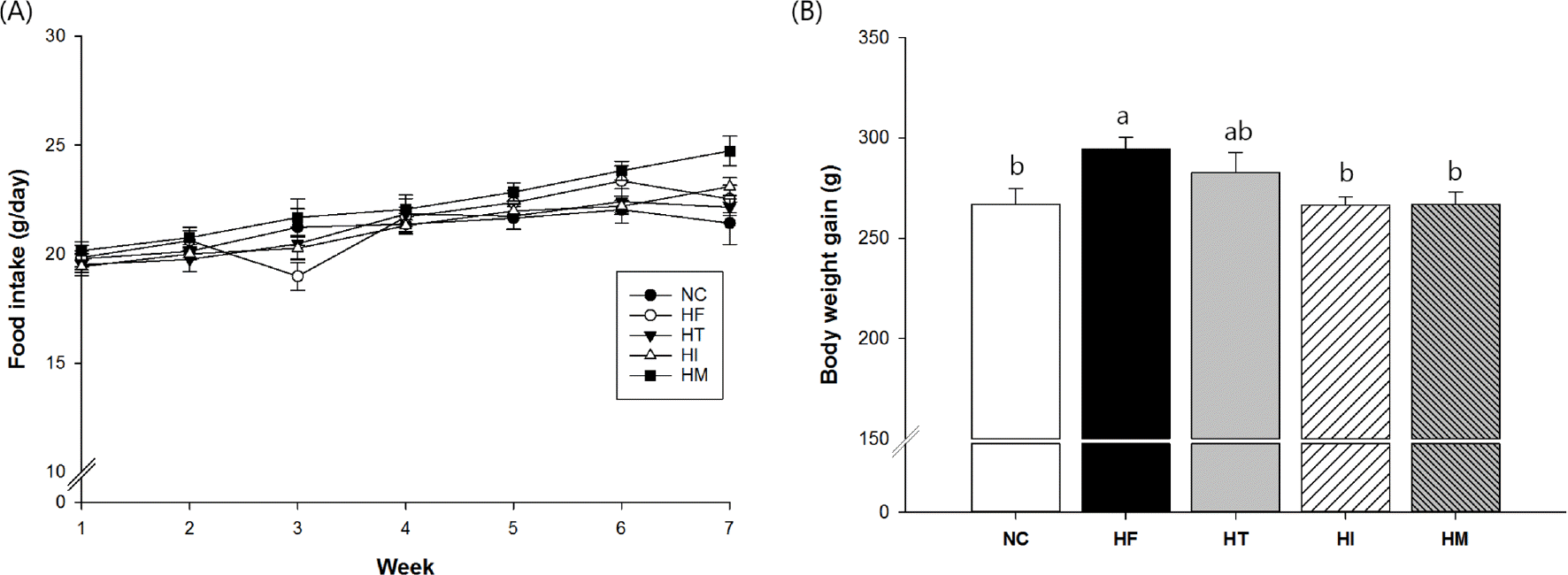
Fig. 2.
Effects of immature and mature persimmon on plasma lipid profiles in rats fed a high-fat/cholesterol diet: (A) lipids (total cholesterol and triglycerides), (B) lipoproteins (VLDL, HDL, and LDL), (C) ox-LDL, and (D) apo-lipoproteins (Apo A and Apo B). NC; normal control, HF; high-fat/cholesterol control, HT; high-fat/cholesterol diet with 1% tannin, HI; high-fat/cholesterol diet with 7% immature persimmon, HM; high-fat/cholesterol diet with 7% mature persimmon. Values are expressed as means ± SE (n = 10 for each group). Means with different letters at each panel are significantly different form each other at p < 0.05 by Duncan's multiple range test.
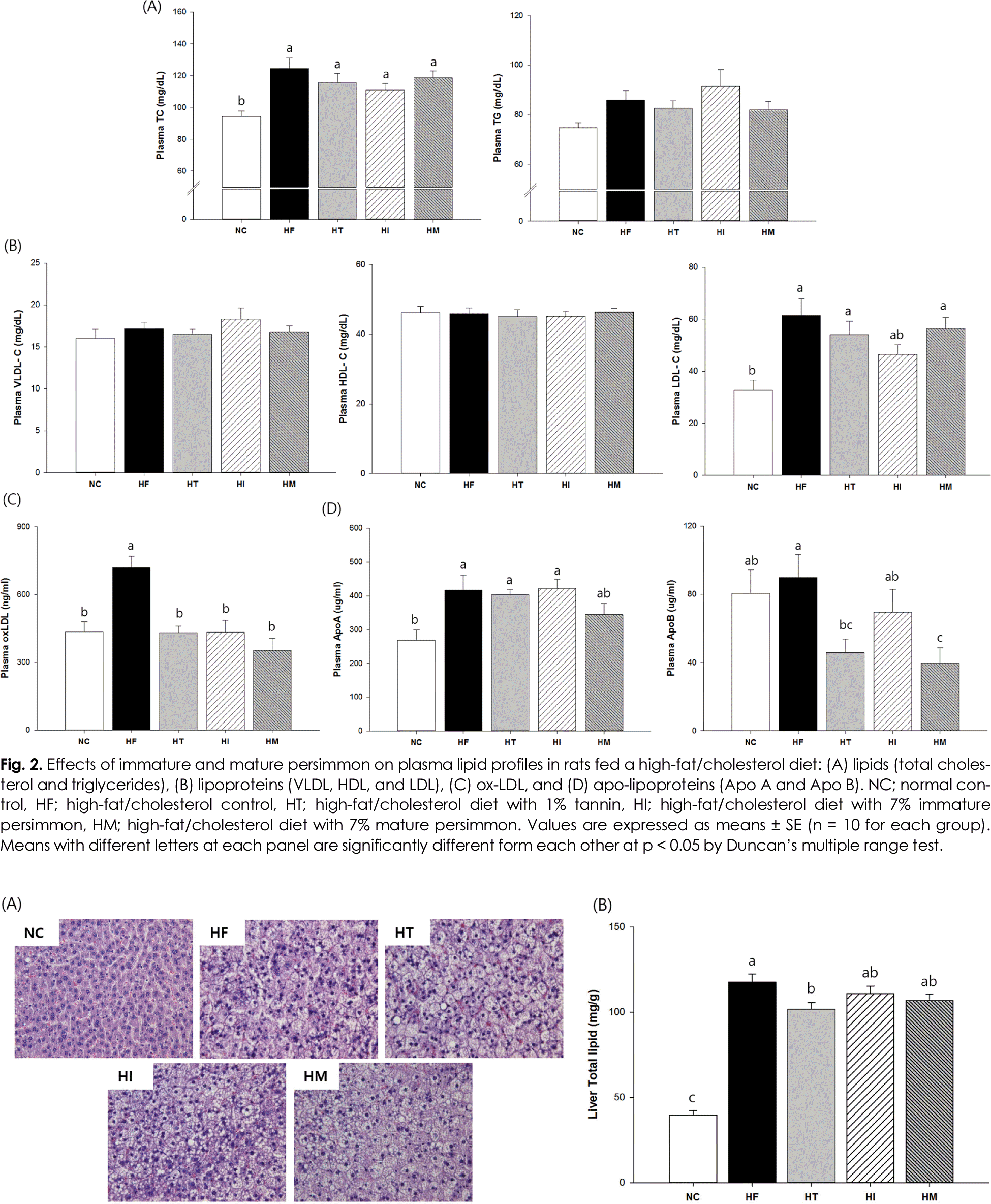
Fig. 3.
Effects of immature and mature persimmon on hepatic histopathology and total lipid content in in rats fed a high-fat/cholesterol diet: (A) hepatic histopathology by H&E staining and (B) total lipid content. NC; normal control, HF; high-fat/cholesterol control, HT; high-fat/cholesterol diet with 1% tannin, HI; high-fat/cholesterol diet with 7% immature persimmon, HM; high-fat/cholesterol diet with 7% mature persimmon. Values are expressed as Means SE (n = 10 for each group). Means with different letters on the bar are significantly different form each other at p < 0.05 by Duncan's multiple range test.
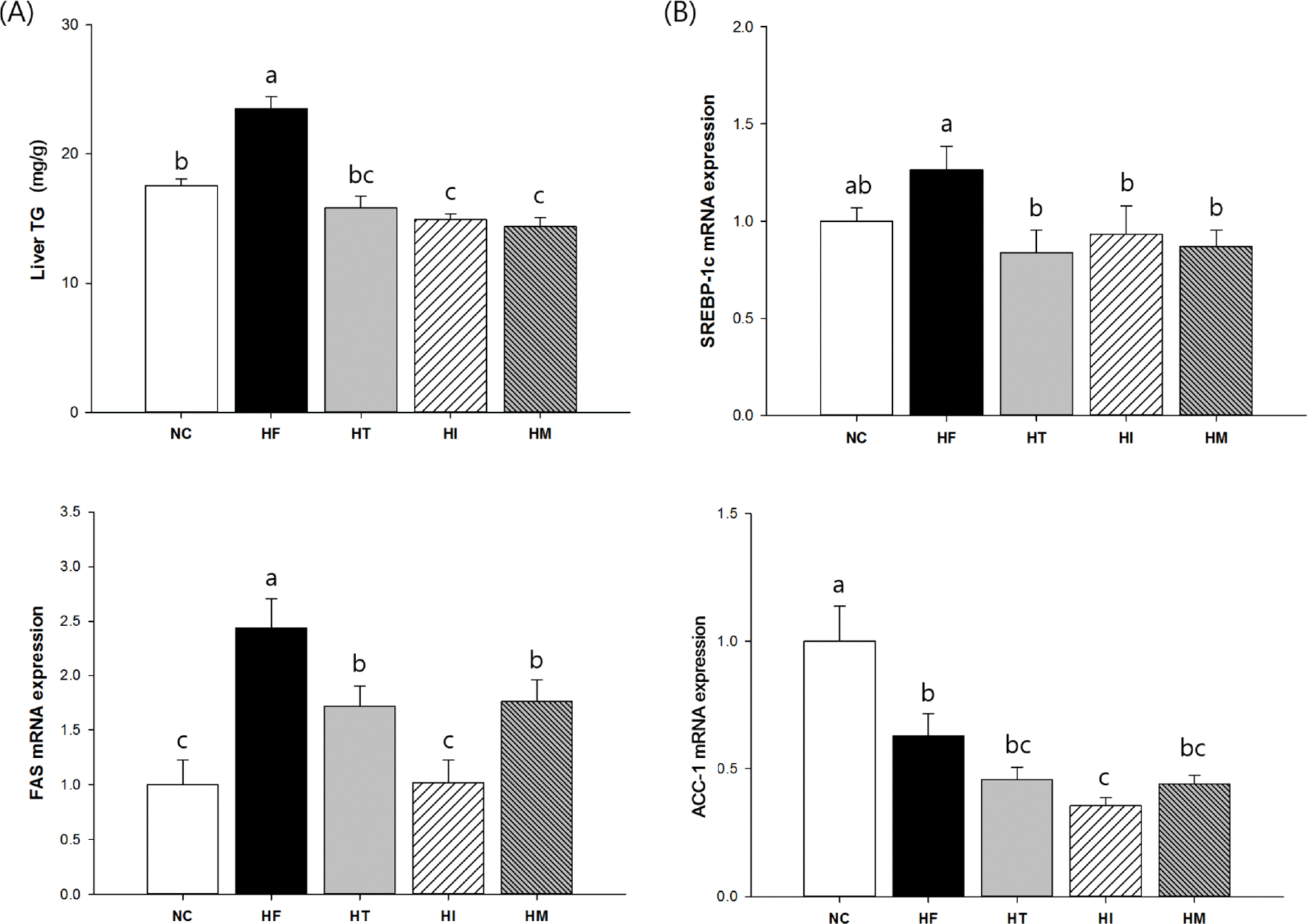
Fig. 4.
Effects of immature and mature persimmon on hepatic triglyceride metabolism in rats fed a high-fat/cholesterol diet: (A) triglycerides and (B) mRNA expressions of SREBP-1c, FAS, and ACC-1 genes. The mRNA expression were normalized to an internal control (GAPDH). NC; normal control, HF; high-fat/cholesterol control, HT; high-fat/cholesterol diet with 1% tannin, HI; high-fat/cholesterol diet with 7% immature persimmon, HM; high-fat/cholesterol diet with 7% mature persimmon. Values are expressed as Means SE (n = 10 for each group). Means with different letters on the bar are significantly different form each other at p < 0.05 by Duncan's multiple range test.
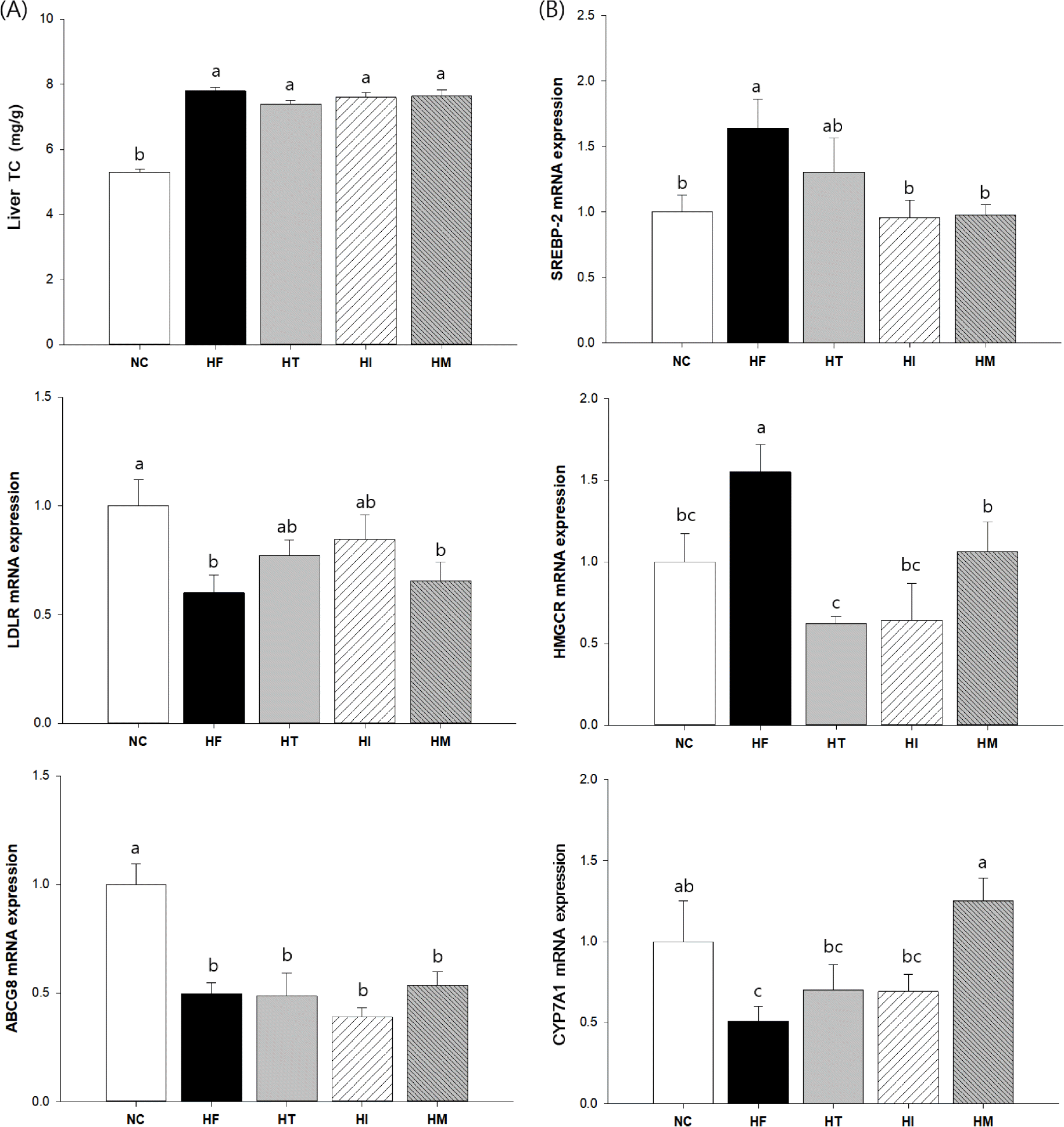
Fig. 5.
Effects of immature and mature persimmon on hepatic cholesterol metabolism regulation in rats fed a high-fat/cholesterol diet: (A) total cholesterol and (B) mRNA expressions of SREBP-2, LDLR, HMGCR, ABCG8, and CYP7A1. The mRNA expressions were normalized to an internal control (GAPDH). NC; normal control, HF; high fat/cholesterol control, HT; high fat/cholesterol diet with 1% tannin, HI; high fat/cholesterol diet with 7% immature persimmon, HM; high fat/cholesterol diet with 7% mature persimmon. Values are expressed as Means SE (n = 10 for each group). Means with different letters on the bar are significantly different form each other at p < 0.05 by Duncan's multiple range test.
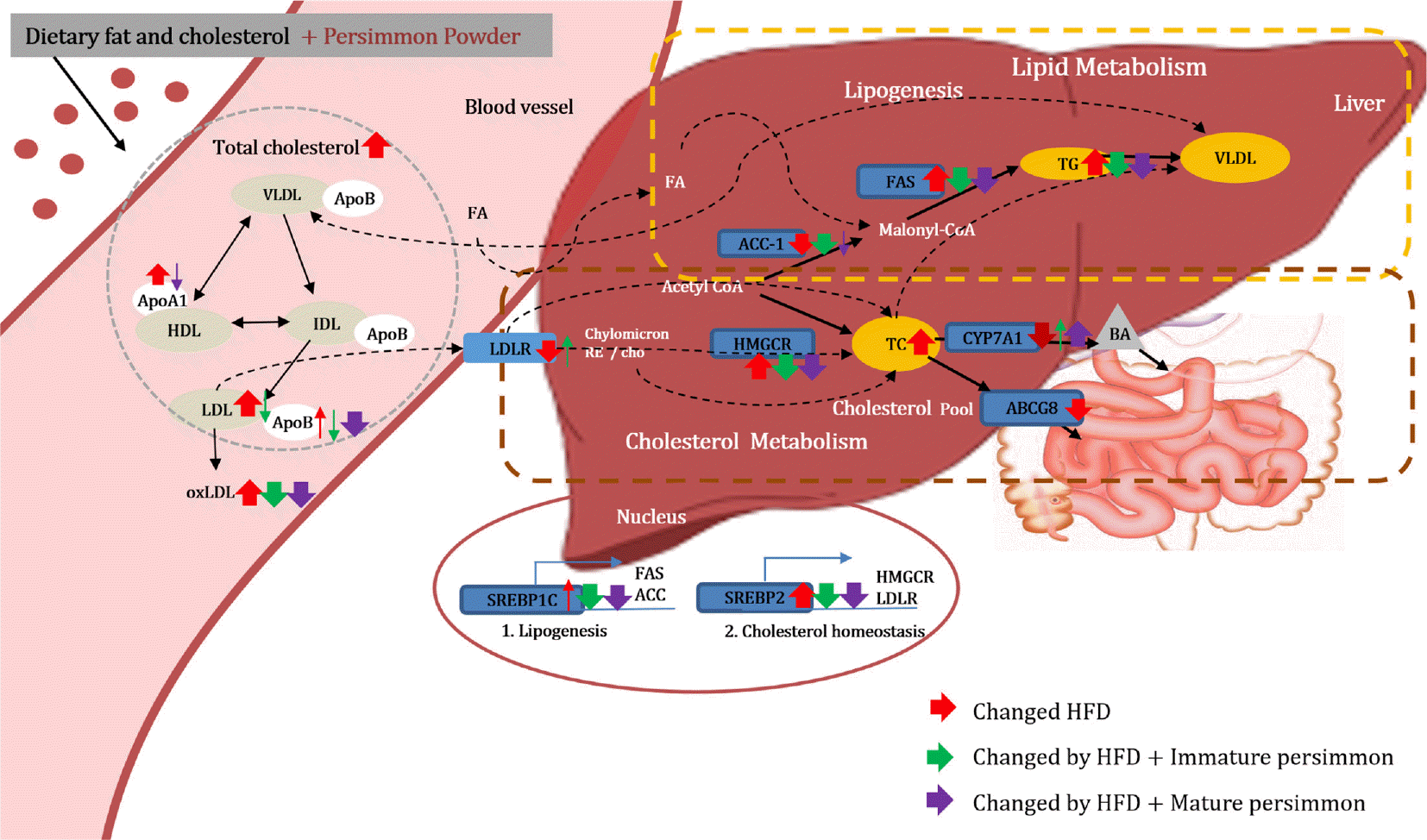
Fig. 6.
Proposed mechanism of immature and mature persimmon on dyslipidemia in rats fed a high-fat/cholesterol diet. VLDL; very low-density lipoprotein, LDL; low-density lipoprotein, HDL; high-density lipoprotein, IDL; intermediate–density lipoprotein, FA; fatty acid, Cho; cholesterol, TG; triglyceride, BA; bile acid, SREBP-1c; sterol regulatory element-binding protein-1c, SREBP-2; sterol regulatory element-binding protein-2, HMGCR; 3-hydroxy-3-methylglutaryl-coenzyme A reductase, LDLR; low density lipoprotein receptor, ABCG8; ATP binding cassette subfamily G member 8, CYP7A1; cytochrome P450 family 7 subfamily A member 1, FAS; fatty acid synthase, ACC-1; Acetyl-CoA carboxylase
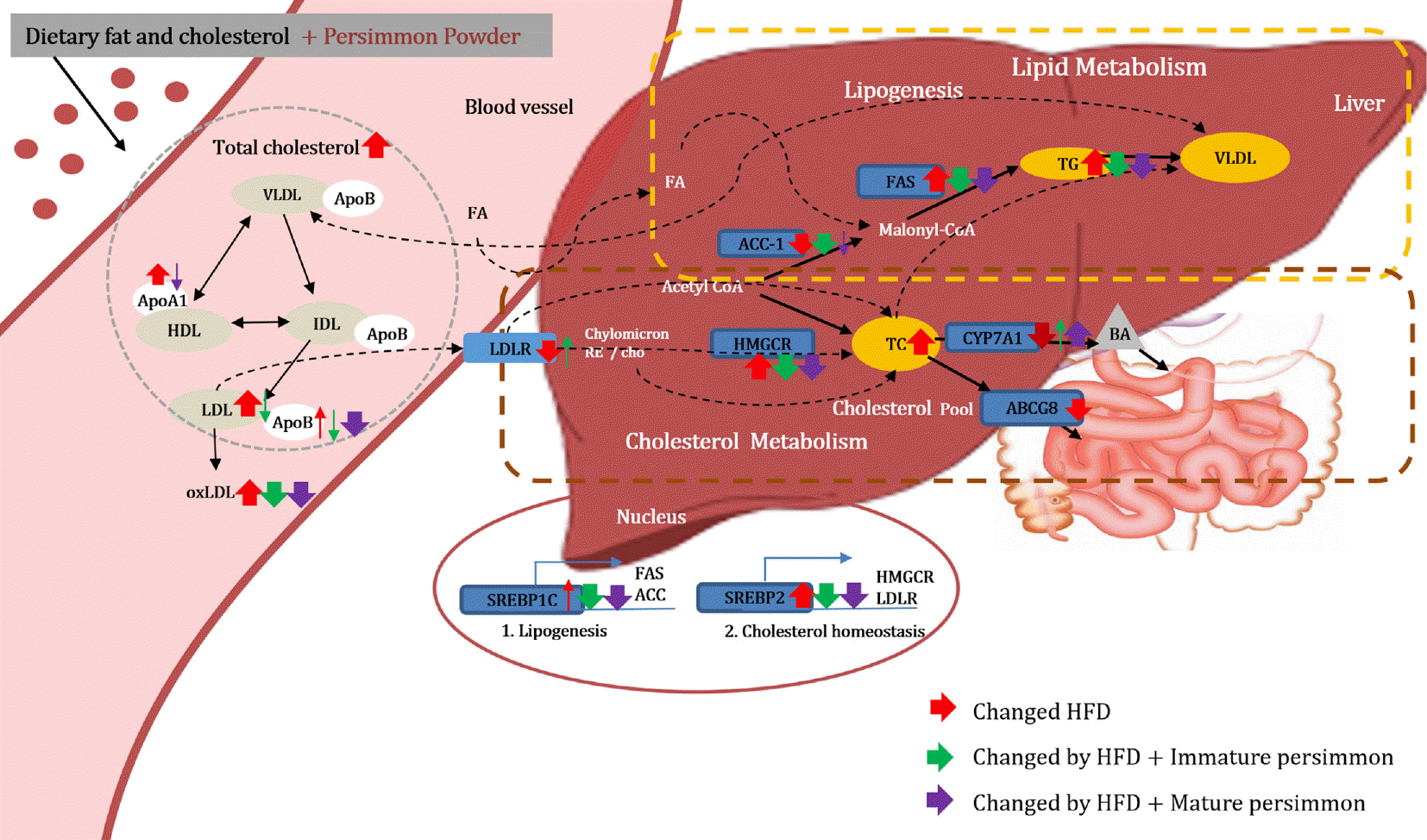
Table 1.
Composition of experimental diet (g)
Table 2.
Primer sequences used for Real-time PCR
1) SREBP-1c, sterol regulatory element-binding protein-1c; SREBP-2, sterol regulatory element-binding protein-2; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; LDLR, low density lipoprotein receptor; ABCG8, ATP binding cassette subfamily G member 8; CYP7A1, cytochrome P450 family 7 subfamily A member 1; FAS, fatty acid synthase; ACC-1, Acetyl-CoA carboxylase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download