Abstract
BACKGROUND/OBJECTIVES
Several pharmacological properties of red rice extract have been reported including anti-oxidant, anti-tumor, and reduced cancer cell invasion. This study was conducted to evaluate the anti-inflammatory effects of red rice extract on the production of inflammatory mediators in lipopolysaccharide (LPS)-induced Raw 264.7 macrophages.
MATERIALS/METHODS
Pro-inflammatory cytokines including tumor necrosis factor-α and interleukin-6 were determined by ELISA and cyclooxygenase-2 and inducible nitric oxide synthase expression was evaluated using western blot analysis. In addition, the signaling pathway controlling the inflammatory cascade such as nuclear factor kappa B (NF-κB), activator proteins-1 (AP-1), and mitogen-activated protein kinase (MAPK) was determined.
RESULTS
Our results showed that red rice polar extract fraction (RR-P), but not non-polar extract fraction, inhibited interleukin-6, tumor necrosis factor-α, and nitric oxide production in LPS-induced Raw 264.7 cells. RR-P also reduced the expression of inflammatory enzymes, inducible nitric oxide synthase, and cyclooxygenase-2. In addition, activation of AP-1 and NF-κB transcription factor in the nucleus was abrogated by RR-P. RR-P inhibited the phosphorylation of extracellular signaling-regulated kinase 1/2, c-Jun NH2-terminal kinase, and p38 MAPK signaling responsible for the expression of inflammatory mediators in LPS-stimulated Raw 264.7 cells. Based on chemical analysis, high amounts of proanthocyanidin and catechins were detected in the RR-P fraction. However, only proanthocyanidin reduced NF-κB and AP-1 activation in LPS-activated Raw 264.7 cells.
Inflammation is an important part of immune pathogenesis and is a response to tissue injury, infection, and stress. Macrophages play key roles in providing an immediate defense against foreign agents [1]. Activation of macrophages has been detected in inflammatory tissue and induced after exposure to tumor necrosis factor-α (TNF-α), interferon-γ, and a microbial lipopolysaccharide (LPS) [2]. During the progression of an inflammatory process, macrophages excessively produced various inflammatory mediators including nitric oxide (NO), prostaglandins, as well as cytokines such as interleukin (IL)-1, IL-6, and TNF-α [3]. However, the prolonged production of inflammatory mediators by macrophage can cause damage to the host and contributes to the pathology of many diseases including arthritis, asthma, cancer, diabetes, and atherosclerosis [4]. Therefore, anti-inflammatory agents are a therapeutic target for healing of inflammation-related diseases.
Activation of the mitogen-activated protein kinases (MAPKs) pathway plays an essential role in the initiation and development of inflammatory processes that are transmitted by sequential phosphorylation events [5]. The MAPKs signaling pathway, including extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38, have been activated by LPS via the Toll like receptor of macrophages [6]. This signaling pathway in turn activates a variety of transcription factors including nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1), which coordinate the induction of a range of inflammatory proteins, such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and cytokines [78]. Hence, any substances that inhibit the activation of NF-κB and AP-1 are considered potential anti-inflammatory agents.
Several biological properties of pigmented rice, such as red and black rice, including anti-oxidant and anti-cancer activities, have been reported [910]. Red and black rice are a potent source of antioxidant in functional foods. Several studies have indicated that the antioxidant capacity of red rice showed higher potency compared with white rice extract [1112]. Antioxidant compounds in red rice were classified as phenolic, flavonoid, vitamin E derivatives, γ-oryzanol, and proanthocyanidin [13]. The antioxidant properties of red rice in terms of scavenging and quenching activity for reactive oxygen species (ROS). Inflammation may be identified as the biological response to oxidative stress. Oxidative stress can lead to chronic inflammation, which in turn could mediate most chronic diseases, including cancer, atherosclerosis, and diabetes [1415]. There is significant evidence indicating that LPS induced mediators of oxidative stress lead to generation of the production of ROS, which themselves induced cytokine production [1617]. Oxidative stress can induce activation of transcription factors including NF-κB and AP-1 which up regulate the expression of inflammatory cytokines [14].
A recent study showed that some natural products could ameliorate antioxidant responses and the inflammatory response in LPS-stimulated macrophages. Some reports have demonstrated that lipophilic phytochemicals contained in rice such as γ-oryzanol, and vitamin E derivatives exert anti-inflammatory activities [1819]. On the other hand, red rice contains high amounts of hydrophilic compounds such as phenolics, flavonoids, and proanthocyanidin. Among those hydrophilic compounds catechin, ferrulic acid, and proanthocyanidin have been proven to exhibit anti-inflammatory activities [202122].
Despite these encouraging studies, reports on the cellular and molecular mechanisms responsible for the anti-inflammatory effect of red rice extract have not been published. Here, we investigated the anti-inflammatory effect of red rice extracts by measuring their ability to inhibit inflammatory mediators and cytokine production. Next, we determined the molecular mechanism underlying the anti-inflammatory impact of red rice extract in LPS-induced Raw 264.7 macrophages.
ELISA kits for detection of IL-6 and TNF-α were purchased from Biolegend (San Diego, CA, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and LPS were obtained from Sigma Aldrich (St Louis, MO, USA). Antibodies for detection of ERK1/2, p38, JNK, c-Jun, p65, and iNOS were purchased from Cell Signaling Technology (Danver, MA, USA). Antibody for detection of COX-2 was purchased from Calbiochem (San Diego, CA, USA). The extraction solvents and the mobile phase of HPLC, including n-butanol, ethanol, and methanol, were purchased from RCI Labscan (Samutsakorn, Thailand).
The grains of red rice (Oryza sativa L.) were harvested from Chiang Mai, Thailand. A voucher specimen number was certified by the herbarium at the Flora of Thailand, Faculty of Pharmacy, Chiang Mai University (voucher specimen no. 023148), which was kept for future reference. To obtain the whole grain of red rice, the grain was dehusked without removing bran and germ. The whole grain was ground using a mortar. The powdered specimens of red rice grains (1.0 kg) were extracted with 50% ethanol by shaking at room temperature overnight. The ethanoic solution was further extracted to determine polar fraction (RR-P) and the remaining rice grains were used to determine the nonpolar fraction (RR-NP). The ethanol was evaporated and the remaining solution was further extracted by shaking with water-saturated butanol in a separating funnel. The butanol was separated and evaporated from the water, and the water fraction was lyophilized and dried to yield the RR-P fraction (0.209% of raw material). For extraction of non-polar compounds including γ-oryzanol and vitamin E derivatives in red rice, the remaining rice grains were extracted with n-butanol by shaking at room temperature overnight. The n-butanol was collected and evaporated. This fraction was lyophilized to yield the RR-NP fraction (0.827% of raw material).
Total phenolic content in red rice extract fractions was measured using Folin-ciocalteu's reagent [23]. Briefly, 300 µL of Folin-ciocalteu was added to the extract. Then, 3 ml of 5% (w/v) Na2CO3 was added to the mixture, followed by incubation for 1 h. The absorbance was measured at 600 nm and gallic acid was used as a standard.
Total proanthocyanidin (condensed tannin) in red rice extract fractions was analyzed using vanillin assay with slight modification, and using catechin as a standard [24]. Red rice extract fractions were reconstituted in sulfuric acid/methanol solution and mixed with 0.1 ml of 1% (w/v) of vanillin in methanol solution. Then 0.1 ml of sulfuric acid (H2SO4) was added, followed by incubation for 15 min in a 30℃ water bath. The absorbance of the sample was measured at 490 nm using a UV-visible spectrophotometer against a reagent blank and compared with a standard curve of catechin at various concentrations. The amount of total proanthocyanidin content in red rice extract was presented as milligram catechin equivalents per gram of extract (mg CE/g extract).
The phenolic compounds, γ-oryzanol and vitamin E derivatives were determined by HPLC using an Inertsil ODS-3-C18 column (phenolic compounds, γ-oryzanol) and HPLC C30 column (vitamin E derivatives) as described in our previous report [10].
Cytotoxicity of red rice extract fractions on Raw 264.7 cells was determined by MTT assay as described previously [25].
Raw 246.7 cells (1 × 106 cells/well) were pre-treated with or without red rice extract fractions (0-200 µg/ml) for 4 h, followed by incubation with 1 µg/ml of LPS for 24 h. Production of TNF-α and IL-6 in the condition media was determined using an ELISA kit (Biolegend Inc, San Diego, CA) according to the manufacturer's instructions [26].
The level of NO in the culture medium was measured using Griess reagent [27]. Raw 264.7 cells (5 × 105 cells/well) were pre-treated with red rice extract fractions (0-200 µg/ml) for 4 h, followed by incubation with or without 1 µg/ml of LPS for 24 h. The supernatant was collected and mixed with an equivalent volume of Griess reagent for 10 min. The absorbance at 550 nm was measured. A standard nitrite curve was generated in the same fashion using sodium nitrite.
Whole cell extraction was performed to determine the expression of COX-2, iNOS, and MAPKs signaling proteins in Raw 246.7 cells. The cells were pretreated with RR-P (0-150 µg/ml) for 4 h, followed by incubation with or without LPS (1 µg/ml) for 24 h or 20 min. The cells were collected and extracted with cell lysis buffer as described previously [25]. For preparation of the nuclear extract fraction, after treating the Raw 246.7 cells with RR-P (0-150 µg/ml) for 4 h, LPS (1 µg/ml) was added to the cells followed by incubation for 30 min at 37 ℃. The treated cells were then collected and washed twice with ice-cold PBS. The nuclear extraction was performed using the NucBusTer protein extraction kit (Novagen, San Diego, CA).
Equal amounts of whole cell lysate or nuclear extract proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and immunoblotted with specific antibodies (anti-COX-2, iNOS, MAPKs, c-Jun, and p65).The levels of β-actin and PARP were used as loading control for whole cell lysate and nucleus protein, respectively [28].
All experiments were performed in triplicate. Quantifications are defined as mean ± SD of three independent experiments and expressed as percentage of the control, which was considered 100%. Throughout this study statistically significant differences were calculated using one-way ANOVA (Prism version 6.0 software). A difference between the experimental groups was considered statistically significant and determined at *P < 0.05, ** P < 0.01, *** P < 0.001 or **** P < 0.0001.
We first examined the cytotoxicity of red rice extract fractions in Raw 264.7 macrophages using an MTT assay. As shown in Fig. 1, RR-P and RR-NP at a high concentration (200 µg/ml) had no effect on cell viability of the macrophages. To examine the effect of red rice extract on the expression level of the inflammatory mediators, Raw 264.7 cells were pretreated with 200 µg/ml of RR-P or RR-NP for 4 h, followed by incubation with LPS for 24 h. As shown in Fig. 2A, only RR-P significantly reduced LPS-stimulated production of IL-6, TNF-α, and NO. In contrast, treatment with RR-NP had no effect on production of LPS-induced inflammatory mediator. In addition, LPS induced production of TNF-α, IL-6, and NO in Raw 246.7 cells was reduced by treatment with RR-P in a dose dependent manner (Fig. 2B).
To determine the effect of RR-P on the expression of iNOS and COX-2, the cells were treated with RR-P (0-150 µg/ml) for 4 h, followed by incubation with LPS for 24 h. As shown in Fig. 3A and B, the expression of iNOS and COX-2 enzymes was almost undetectable in unstimulated Raw 264.7 cells. However, after LPS stimulation, the levels of iNOS and COX-2 were significantly increased and pretreatment of macrophages with RR-P resulted in significantly reduced LPS-induced expression of iNOS and COX-2 in a dose dependent manner.
To further determine transcriptional control of RR-P in Raw 264.7 cells, we examined the effects of RR-P on the level of AP-1 (c-jun) and NF-κB (p-65) in the cell nucleus (Fig. 4). Treatment of Raw 264.7 cells with LPS alone induced the level of c-Jun and p65 in the nuclear extract and pretreatment of the cells with RR-P resulted in dramatic attenuation of the level of c-Jun and p65.
We determined the effect of RR-P on LPS-induced phosphorylation of ERK1/2, p-38, and JNK in Raw 264.7 cells. As shown in Fig. 5, phosphorylation of MAPKs was increased after LPS stimulation. However, pretreatment of the cells with RR-P resulted in significantly reduced LPS-induced phosphorylation of ERK1/2, p-38, and JNK in a dose dependent manner, while RR-P had no effect on the level of the non-phosphorylated forms of ERK1/2, p-38, and JNK. These results demonstrated that RR-P regulated LPS-induced phosphorylation of MAPKs signaling in macrophage cells.
As shown in Table 1, the RR-P extract fraction had a high content of total phenolic and proanthocyanidin. In contrast, low levels of γ-oryzanol, tocotrienols, and tocopherols were detected in RR-P. On the other hand, high concentrations of γ-oryzanol, γ-tocopherol, and γ-tocotrienol were detected in the RR-NP extract fraction (Table 2). RR-NP extract fraction presented trace amounts of total phenolic. The phenolic compounds in the red rice fraction were further identified by HPLC. As shown in Table 3, the RR-P fraction contained a high content of catechin, followed by hydroxybenzoic acid and vanilic acid and low amounts of ferulic acid, protocatechuic acid, and coumaric acid.
The main phytochemicals in RR-P, catechins, and proanthocyanidin were investigated to determine the phytochemicals present in RR-P responsible for the anti-inflammatory property. Raw 264.7 cells were pretreated with 50 µg/ml of proanthocyanidin and catechin for 4 h, followed by treatment with LPS for 30 min. The nuclear extracts were used to determine the level of two critical transcription factors in the inflammatory response, NF-κB and AP-1. As shown in Fig. 6, proanthocyanidin reduced LPS-induced activation of NF-κB and AP-1. In contrast, catechins had no effect on LPS-induced activation of AP-1 and NF-κB.
In the current study, we reported for the first time that the RR-P fraction exerts anti-inflammatory activities by inhibiting the production of TNF-α, IL-6, and NO in LPS-activated macrophages. In contrast, the RR-NP fraction had no effect on LPS-induced inflammation in macrophages. In addition, RR-P suppressed the LPS-stimulated expression of iNOS and COX-2 protein in Raw 264.7 cells. These findings suggest that the RR-P fraction possesses useful anti-inflammatory activities by regulation of pro-inflammatory mediators and cytokines.
Many studies have shown that the expression of inflammatory proteins is regulated not only by NF-κB [2930] but also by other transcription factors, including AP-1 [31]. The most common component member of AP-1 is the c-Jun/c-Fos heterodimers that bind to the TPA-response elements (AP-1 site) in the promoter of various genes. In this study, we found that RR-P inhibited AP-1 and NF-κB activity by decreasing the nuclear level of c-Jun and p65, respectively. These findings are consistent with those of studies showing that the AP-1 and NF-κB response elements are present in the promoters, which can regulate COX-2, iNOS, TNF-α, and IL-6 genes.
Phosphorylation of MAPKs signaling proteins, p38, ERK1/2, and JNK, has been shown to initiate inflammatory gene expression in LPS-induced macrophages. Recent studies have indicated that the inhibition of LPS-induced MAPK signaling pathways results in the inactivation of NF-κB and AP-1 [32]. To determine whether the inhibition of NF-κB and AP-1 activation by RR-P is mediated through the MAPK pathway, the LPS-induced phosphorylation of p38, JNK, and ERK1/2 was assessed in Raw 264.7 cells. The results showed that the phosphorylation of p38, JNK, and ERK1/2 by LPS was inhibited by RR-P treatment. Thus, it is likely that the inhibition of MAPKs phosphorylation by RR-P may contribute to the RR-P mediated inhibition of NF-κB and AP-1 in LPS-induced macrophages.
Pigmented rice contains a variety of bioactive components with anti-inflammation activities [33]. However, no studies have reported on the anti-inflammatory effect of red rice extract, and the active compound associated with anti-inflammation in red rice has not been investigated. To minimize the toxicity of organic extraction solvents, alcohol base solvents including ethanol and butanol were used in this study. In the report from Jun HI et al., rice brans extracted with an aqueous mixture of ethanol showed a higher content of phenolic and flavonoid compounds than absolute ethanol [34]. In addition, 50% ethanol has been used in extraction of phenolic, flavonoids, and proanthocyanidin from different types of seeds [3536]. Therefore, we used 50% ethanol as the first solvent for extraction of the hydrophilic phytochemical compounds from red rice. On the other hand, ethyl acetate is the best solvent for extraction of phenolic and flavonoids. However, the polarity of butanol is close with ethyl acetate but with less toxicity. Thus, we used water-saturated butanol to further purified phenolic, flavonoids, and proanthocyanidin from the 50% ethanoic fraction and this extract was called RR-P. Srisaipet A et al. reported that n-butanol has been used in extraction of γ-oryzanol and vitamin E derivatives from rice bran oils comparable with hexane [37]. Here in, n-butanol was used in extraction of hydrophobic phytochemical from the remaining rice grains after 50% ethanoic extract and this n-butanol extract was called RR-NP.
Our results demonstrated that RR-P was rich in phenolic compounds and proanthocyanidins, while lower amounts or none at all were detected in RR-NP. The main phenolic component in RR-P was a catechin followed by hydroxybenzoic acid and vanilic acid. It is rational to speculate that catechins and proanthocyanidins might contribute to the anti-inflammation function of RR-P. In this study, we found that proanthocyanidins at 50 µg/ml reduced the activities of AP-1 and NF-κB, while catechins at 50 µg/ml had no effect. This result was similar to certain previous findings, which showed that proanthocyanidin derived from blackberries inhibited the inflammatory mediator in LPS-induced Raw 264.7 cells [38]. In addition, treatment of proanthocyanidins from Ephedra root reduced TNF-α and IL-1-β via inhibited NF-κB activity [39]. Our data collectively support the acknowledgement of the inhibitory effect of RR-P on anti-inflammation activity, which might be the effect of proanthocyanidin. On the other hand, the content of γ-oryzanol, tocotrienols, and tocopherols was detected in RR-NP fractions. Specifically, γ-oryzanol is the major component of the RR-NP fraction. Results of this study indicated that RR-NP did not exhibit an obvious anti-inflammatory activity at the concentration of up to 200 µg/ml. This finding was in contrast with the study by Saenjum et al., which reported that γ-oryzanol-rich extract from Thai rice, prepared and semi-purified by column chromatography, reduced NO production in LPS-induced Raw 264.7 cells [40]. However, a high concentration (IC50; 29.3 µg/ml) of the extract was used to achieve the anti-inflammation activity. Although suppression of LPS-induced inflammation by pure tocotrienols and tocopherols (8-16 µM) has been reported, the concentrations of tocotrienols and tocopherols present in RR-NP (< 1 µmol/l) were not sufficient doses to promote anti-inflammatory activity [41]. In addition, a recent study reported that γ-tocopherol or γ-tocotrienol inhibited LPS-stimulated production of IL-6 without having an effect on TNF-α, IL-10, and COX-2 in Raw 264.7 cells [42]. These observations are consistent with our findings showing that RR-NP had no significant effect on LPS-stimulated increases of TNF-α and on NO production. This could imply that the concentrations of the bioactive component in RR-NP may not reach their potential to inhibit LPS-induced inflammation.
In conclusion, this study has shown that RR-P inhibited the production of NO, IL-6, TNF-α, COX-2, and iNOS in LPS-treated Raw 264.7 cells. The inhibitory effects are mediated by the inhibition of AP-1, NF-κB activation and the MAPKs signaling pathway. The anti-inflammation activity of the RR-P fraction may be due in part to the effect of the proanthocyanidin. These results also suggest that the RR-P fraction of red rice may be a potent natural anti-inflammatory agent.
Figures and Tables
Fig. 1
Effect of red rice extract fractions on the viability of Raw 264.7 cells.
The cells were seeded into 96-well plates and treated with RR-P or RR-NP for 48 h. The cell viability was determined by MTT assay. The results are expressed as percentages of cell viability relative to the untreated cells and presented as the mean ± SD of three independent experiments.
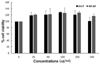
Fig. 2
Effect of red rice extract fraction on the level of IL-6, TNF-α, and NO production in LPS-induced Raw 264.7 cells.
The cells were pre-treated with 200 µg/ml of RR-P or RR-NP for 4h, followed by treatment with LPS for 24h. The levels of IL-6 and TNF-α were determined with ELISA and NO production was assayed using Griess reagent (A). The effects of various concentrations of RR-P (0-200 µg/ml) on the level of IL-6, TNF-α, and NO in LPS-induced Raw macrophages are presented (B). Data were obtained from three independent experiments and expressed as mean ± SD. Significant differences from the LPS induction group are depicted by * P < 0.05, ** P < 0.01, or **** P < 0.0001.

Fig. 3
RR-P reduced the expression of iNOS and COX-2 in LPS-induced Raw 264.7 cells.
The cells were pretreated with RR-P for 4 h before stimulation with or without LPS for 24 h. The expression levels of iNOS (A) and COX-2 (B) in the cells were determined by western blot analysis. Relative densities were calculated as the ratio of iNOS and COX-2 expression to β-actin levels. Data are represented as the mean ± SD of three experiments. * P < 0.05, *** P < 0.001 or **** P < 0.0001 compared to LPS alone.
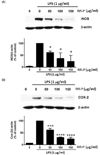
Fig. 4
Effect of RR-P on the level of NF-κB and AP-1 in the nucleus of LPS-induced Raw macrophages.
The cells were pretreated with RR-P for 4 h, and stimulated with LPS for 30 min. The cell nucleus fraction was prepared and the level of NF-κB and AP-1 (c-Jun) was determined by western blot analysis. Relative densities were determined as the ratio of NF-κB expression to PARP level and c-Jun expression to PARP level. Data are represented as the mean ± SD of three experiments. * P < 0.05 or *** P < 0.001 compared to LPS alone.
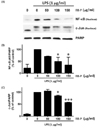
Fig. 5
Effect of RR-P on LPS induced phosphorylation of MAPK in Raw 264.7 cells.
The cells were pretreated with RR-P for 4 h, and stimulated with LPS for 20 min. The whole cells lysate were prepared for measurement of phosphorylated and non-phosphorylated forms of ERK1/2, p-38, and JNK by western blot analysis (A). Relative protein levels were quantified by scanning densitometry and normalized to control protein levels (B). Data are presented as the mean ± SD of three experiments. ** P < 0.01, *** P < 0.001, or **** P < 0.0001 compared to LPS alone.

Fig. 6
Effect of proanthocyanidin and catechin on LPS induced NF-κB and AP-1activities in Raw 264.7 cells.
The cells were pretreated with 50 µg/ml of proanthocyanidin or catechin for 4 h and stimulated with LPS for 30 min. The nucleus-extracted fraction was prepared and used for determining NF-κB and AP-1 (c-Jun) levels by western blot analysis. Relative densities were determined as the ratio of NF-κB expression to PARP level and c-Jun expression to PARP level. Data are presented as the mean ± SD of three experiments. * P < 0.05 or ** P < 0.01 compared to LPS alone.
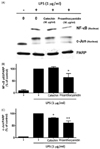
Table 1
The total phenolic, γ-oryzanol, and proanthocyanidin contents in red rice extract fractions.

Notes
This research study was supported financially by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, the Agricultural Research Development Agency (Public Organization) (ARDA) and the National Research Council of Thailand (NRCT).
References
1. Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008; 214:161–178.

2. Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for tumour necrosis factor alpha. Biochem J. 1999; 342:21–25.

3. Han S, Lee JH, Kim C, Nam D, Chung WS, Lee SG, Ahn KS, Cho SK, Cho M, Ahn KS. Capillarisin inhibits iNOS, COX-2 expression, and proinflammatory cytokines in LPS-induced RAW 264.7 macrophages via the suppression of ERK, JNK, and NF-kappaB activation. Immunopharmacol Immunotoxicol. 2013; 35:34–42.

4. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015; 160:816–827.

5. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy - from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005; 1754:253–262.

6. Zhang Y, Yan R, Hu Y. Oxymatrine inhibits lipopolysaccharide-induced inflammation by down-regulating Toll-like receptor 4/nuclear factor-kappa B in macrophages. Can J Physiol Pharmacol. 2014; 93:253–260.

7. Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappa B-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013; 218:1452–1467.

8. Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, Lee DU, Kim YS. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012; 14:375–383.

9. Kim GR, Jung ES, Lee S, Lim SH, Ha SH, Lee CH. Combined mass spectrometry-based metabolite profiling of different pigmented rice (Oryza sativa L.) seeds and correlation with antioxidant activities. Molecules. 2014; 19:15673–15686.

10. Pintha K, Yodkeeree S, Pitchakarn P, Limtrakul P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac J Cancer Prev. 2014; 15:4601–4607.

11. Laokuldilok T, Shoemaker CF, Jongkaewwattana S, Tulyathan V. Antioxidants and antioxidant activity of several pigmented rice brans. J Agric Food Chem. 2011; 59:193–199.

12. Muntana N, Prasong S. Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pak J Biol Sci. 2010; 13:170–174.

13. Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci Nutr. 2014; 2:75–104.

14. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49:1603–1616.

15. Lakkur S, Judd S, Bostick RM, McClellan W, Flanders WD, Stevens VL, Goodman M. Oxidative stress, inflammation, and markers of cardiovascular health. Atherosclerosis. 2015; 243:38–43.

16. Wang JS, Wu D, Huang DY, Lin WW. TAK1 inhibition-induced RIP1-dependent apoptosis in murine macrophages relies on constitutive TNF-alpha signaling and ROS production. J Biomed Sci. 2015; 22:76.
17. Choudhury S, Ghosh S, Gupta P, Mukherjee S, Chattopadhyay S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-kappaB and SAPK/JNK pathway. Free Radic Res. 2015; 49:1371–1383.

18. Mizushina Y, Kuriyama I, Yamazaki A, Akashi T, Yoshida H. Cycloartenyl trans-ferulate, a component of the bran byproduct of sake-brewing rice, inhibits mammalian DNA polymerase and suppresses inflammation. Food Chem. 2013; 141:1000–1007.

19. Ng LT, Ko HJ. Comparative effects of tocotrienol-rich fraction, alpha-tocopherol and alpha-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012; 134:920–925.

20. Lyu SY, Park WB. Production of cytokine and NO by and PBMC in vitro incubation with RAW 264.7 macrophages flavonoids. Arch Pharm Res. 2005; 28:573–581.

21. Ronchetti D, Borghi V, Gaitan G, Herrero JF, Impagnatiello F. NCX 2057, a novel NO-releasing derivative of ferulic acid, suppresses inflammatory and nociceptive responses in in vitro and in vivo models. Br J Pharmacol. 2009; 158:569–579.

22. Ahmad SF, Zoheir KM, Abdel-Hamied HE, Ashour AE, Bakheet SA, Attia SM, Abd-Allah AR. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int Immunopharmacol. 2013; 17:79–87.

23. Zhang H, Shao Y, Bao J, Beta T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015; 172:630–639.

24. Gunaratne A, Wu K, Li D, Bentota A, Corke H, Cai YZ. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013; 138:1153–1161.

25. Wongsirisin P, Yodkeeree S, Pompimon W, Limtrakul P. Induction of G1 arrest and apoptosis in human cancer cells by crebanine, an alkaloid from Stephania venosa. Chem Pharm Bull (Tokyo). 2012; 60:1283–1289.

26. Lee J, Nakagiri T, Kamimura D, Harada M, Oto T, Susaki Y, Shintani Y, Inoue M, Miyoshi S, Morii E, Hirano T, Murakami M, Okumura M. IL-6 amplifier activation in epithelial regions of bronchi after allogeneic lung transplantation. Int Immunol. 2013; 25:319–332.

27. Kwon DJ, Ju SM, Youn GS, Choi SY, Park J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-kappaB and AP-1 activation in RAW 264.7 macrophages. Food Chem Toxicol. 2013; 58:479–486.

28. Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009; 69:6581–6589.

29. Choi RJ, Chun J, Khan S, Kim YS. Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells. Int Immunopharmacol. 2014; 18:182–190.

30. Lu Y, Suh SJ, Kwak CH, Kwon KM, Seo CS, Li Y, Jin Y, Li X, Hwang SL, Kwon O, Chang YC, Park YG, Park SS, Son JK, Kim CH, Chang HW. Saucerneol F, a new lignan, inhibits iNOS expression via MAPKs, NF-kappaB and AP-1 inactivation in LPS-induced RAW264.7 cells. Int Immunopharmacol. 2012; 12:175–181.

31. Kwon JY, Hong SH, Park SD, Ahn SG, Yoon JH, Kwon BM, Kim SA. 2'-Benzoyloxycinnamaldehyde inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells via regulation of AP-1 pathway. Eur J Pharmacol. 2012; 696:179–186.

32. Khan S, Shehzad O, Lee KJ, Tosun A, Kim YS. Anti-inflammatory properties of samidin from Seseli resinosum through suppression of NF-kappa B and AP-1-mediated-genes in LPS-stimulated RAW 264.7 cells. Arch Pharm Res. 2014; 37:1496–1503.

33. Jang S, Xu Z. Lipophilic and hydrophilic antioxidants and their antioxidant activities in purple rice bran. J Agric Food Chem. 2009; 57:858–862.

34. Jun HI, Song GS, Yang EI, Youn Y, Kim YS. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J Food Sci. 2012; 77:C759–C764.

35. Burdette A, Garner PL, Mayer EP, Hargrove JL, Hartle DK, Greenspan P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J Med Food. 2010; 13:879–887.

36. Inglett GE, Chen D, Rose DJ, Berhow M. High-shear, jet-cooking, and alkali treatment of corn distillers' dried grains to obtain products with enhanced protein, oil and phenolic antioxidants. Food Sci Technol Int. 2010; 16:297–304.

37. Srisaipet A, Nuddagul M. Influence of temperature on gamma-oryzanol stability of edible rice bran oil during heating. Int J Chem Eng Appl. 2014; 5:303–306.

38. Cuevas-Rodríguez EO, Dia VP, Yousef GG, García-Saucedo PA, López-Medina J, Paredes-López O, Gonzalez de, Lila MA. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J Agric Food Chem. 2010; 58:9542–9548.

39. Kim IS, Park YJ, Yoon SJ, Lee HB. Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor-kappa B activation in RAW 264.7 macrophages. Int Immunopharmacol. 2010; 10:1616–1625.

40. Chalermpong S. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J Med Plant Res. 2012; 6:1070–1077.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download