Abstract
BACKGROUND/OBJECTIVES
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, involves chronic inflammation of the gastrointestinal tract. Previously, Sasa quelpaertensis leaves have been shown to mediate anti-inflammation and anti-cancer effects, although it remains unclear whether Sasa leaves are able to attenuate inflammation-related intestinal diseases. Therefore, the aim of this study was to investigate the anti-inflammatory effects of Sasa quelpaertensis leaf extract (SQE) using an in vitro co-culture model of the intestinal epithelial environment.
MATERIALS/METHODS
An in vitro co-culture system was established that consisted of intestinal epithelial Caco-2 cells and RAW 264.7 macrophages. Treatment with lipopolysaccharide (LPS) was used to induce inflammation.
RESULTS
Treatment with SQE significantly suppressed the secretion of LPS-induced nitric oxide (NO), prostaglandin E2 (PGE2), IL-6, and IL-1β in co-cultured RAW 264.7 macrophages. In addition, expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and tumor necrosis factor (TNF)-α were down-regulated in response to inhibition of IκBα phosphorylation by SQE. Compared with two bioactive compounds that have previously been identified in SQE, tricin and P-coumaric acid, SQE exhibited the most effective anti-inflammatory properties.
CONCLUSIONS
SQE exhibited intestinal anti-inflammatory activity by inhibiting various inflammatory mediators mediated through nuclear transcription factor kappa-B (NF-kB) activation. Thus, SQE has the potential to ameliorate inflammation-related diseases, including IBD, by limiting excessive production of pro-inflammatory mediators.
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn's disease (CD), and these diseases are characterized by chronic inflammation of the gastrointestinal tract. However, the etiopathogenesis for these diseases has not been elucidated [1,2]. In addition, the prevalence of UC and CD has been increasing worldwide. The clinical features of CD include pain, diarrhea, and narrowing of the gut lumen which causes strictures and fistulization of the skin that lead to bowel obstruction [3]. The clinical features of UC include an increasing loss of peristaltic function, diarrhea, blood loss, and bloody stool [4]. During the propagation and initiation of IBD, the mucosal epithelial barrier is compromised and macrophages secrete chemokines and pro-inflammatory cytokine [5,6]. In patients with IBD, intestinal immune cells and macrophages have been found to secrete large amounts of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, prostaglandin E2 (PGE2), and tumor necrosis factor (TNF)-α, thereby leading to damage of the intestinal epithelial monolayers and subsequent mucosal inflammation [7,8]. Nuclear transcription factor kappa-B (NF-κB) regulates various inflammation-related genes, and is activated and translocates into the nucleus via phosphorylation of the IκB complex. Once in the nucleus, NF-κB binds the IκB promoter region of various target genes and induces the production of pro-inflammatory mediators such as cyclooxygenase-2 (COX-2) inducible nitric oxide synthase (iNOS), TNF-α, IL-6, and IL-1β [9,10].
Previously, intestinal inflammation has been studied using animal models. The most widely used animal models for colitis include dextran sodium sulfate (DSS), and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced models [11]. There are also several genetically modified animal models that are available, including an IL-2 knock-out mouse [12], an IL-10 knock-out mouse [13], and an IL-15 transgenic mouse [14]. However, in vitro models are also needed to understand the regulatory mechanisms of anti-inflammatory drugs or food factors in colitis. In the present study, an in vitro model of intestinal inflammation was established using a co-culture system of human intestinal epithelial-like Caco-2 cells and RAW 264.7 macrophages. This model closely resembles human colitis at the cellular level, and thus, was used to investigate the effects of Sasa quelpaertensis leaf extract (SQE) in comparison with individual bioactive compounds of SQE, tricin and P-coumaric acid.
Interest in identifying new functional food resources from herbal medicines used to treat various gastrointestinal diseases, including IBD, has recently increased [15]. The genus Sasa (poaceae), known as bamboo grass, is broadly cultivated in Korea, China, Japan, and Russia [16]. Moreover, Sasa leaves are commonly used for the treatment of hypertension, cardiovascular disease, inflammation, and cancer [17,18,19]. Sasa quelpaertensis Nakai is a bamboo grass native to Korea and is only cultivated on Mt. Halla on Jeju Island in South Korea. Analysis of SQE has previously showed that it contains a mixture of polysaccharides, amino acids, and polyphenols, including tricin and P-coumaric acid [19]. Although several studies have reported a number of beneficial effects mediated by Sasa leaves, only a limited number of studies have investigated the anti-inflammatory effects and mechanisms of SQE on chronic colitis at the cellular level in vitro.
Therefore, in the present study, SQE was prepared and evaluated for its potential to mediate anti-inflammatory effects. In addition, the effects of SQE on related inflammatory mediators and NF-κB signaling in response to LPS in co-cultured Caco-2 cells and RAW 264.7 macrophages was investigated.
Sasa quelpaertensis leaves were obtained from Jeju Island in South Korea. After being cleaned and dried, leaves (1 kg) were mixed with ethanol:water (70:30, v/v) and were incubated at room temperature (RT) for 48 h. The resulting SQE was filtered and concentrated at 60℃ using a rotary evaporator, then was freeze-dried and ground into a powder. SQE was stored at -20℃ until needed. A 2695 high performance liquid chromatography (HPLC) Alliance system (Waters Corp., Milford, MA, USA) was used to analyze the major compounds present in the SQE, including P-coumaric acid and tricin. HPLC system included a column oven, auto-injector, pump and Waters 2998 photodiode array detector. A detailed analysis of SQE and its chromatogram has been described elsewhere and both P-coumaric acid (1.13 mg/g) and tricin (0.82 mg/g) were identified as peaks in the HPLC chromatograms [20].
The human intestinal epithelial cell line, Caco-2 cells, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), and was cultured in high glucose Dubelcco's modified Eagle's medium (DMEM) (Gibco, Rockville, MD, USA) supplemented with Non-Essential Amino Acids (Invitrogen, Carlsbad, CA, USA). Murine macrophage cells, RAW 264.7, were obtained from the Korean Cell Line Bank and were maintained in DMEM with 100 µg/ml streptomycin, 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA), and 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) at 37℃ in a humidified 5% CO2 atmosphere.
RAW 264.7 macrophages were seeded in 12-well plates (5.0 × 105cells/well) and incubated at 37℃ with 5% CO2. For cytotoxicity assays, cells were treated with various concentrations of SQE (100, 200, and 400 µg/ml) or comparable doses of tricin (1 µM) and P-coumaric acid (2.4 µM). After 24 h, the cells were trypsinized, pellets were washed with PBS, and each set of cells was resuspended. The cells were subsequently stained with trypan blue and unstained viable cells were counted using a hemocytometer. The proportion of unstained cells to the total number of cells were recorded and used to calculate cell viability.
A co-culture system was established as described previously [21]. Briefly, Caco-2 cells (passage numbers: 48 - 62) were seeded onto Transwell insert plates (3.75 × 105 cells/well; Corning Costar Corp., Cambridge, MA, USA) and incubated for 21 d to obtain an integrated cell monolayer with a transepithelial electrical resistance value (TER) 1,200 Ω cm2 using a Millicell-ERS instrument (Millipore Corporation, Billerica, MA, USA). Cell culture medium was changed every 3 d. RAW 264.7 macrophages (passage numbers: 10 - 30) were seeded in 6-well tissue culture plates (8.5 × 105 cells/well) and incubated overnight. After replacing the media with RPMI1640, transwell inserts containing Caco-2 cells were added to the 6-well plates containing RAW 264.7 macrophages (Fig. 1). To evaluate the anti-inflammatory effects of SQE in this co-culture system, various concentrations of SQE were applied to the apical side of the transwell insert for 3 h, followed by the addition of 1 µg/ml lipopolysaccharide (LPS, Santa Cruz Biotechnology, Santa Cruz, CA, USA) to the basolateral side. After an additional incubation overnight, culture supernatants from the basolateral side were collected to measure levels of nitric oxide (NO), PGE2, IL-6, and IL-1β. The cultured cells were subsequently harvested to isolate total RNA for RT-PCR and western blot analyses.
NO production was monitored by measuring the nitrite content in RAW 264.7 macrophages culture medium. Briefly, Griess reagent (0.1% N-1-naphthylenediamine dihydrochloride and 5% H3PO4 solution) was added to each sample in a 1:1 (v/v) manner. After gentle mixing and 15 min incubation in the dark, NO levels were subsequently measured and compared with a nitrate standard curve. Absorbance values at 560 nm were measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Intestinal epithelial Caco-2 cells and RAW 264.7 macrophages were co-cultured in the presence of either LPS (1 µg/ml) alone or LPS in combination with SQE (100, 200, or 400 µg/ml), tricin, or P-coumaric acid. Each culture medium was collected and the levels of PGE2, IL-6, and IL-1β were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA).
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and total protein was extracted using radioimmunoprecipitation (RIPA) buffer. Following centrifugation (13,000 × g, 15 min, 4℃), supernatants were collected and stored at -80℃. The total protein concentration for each sample was determined using the Bradford colorimetric method [22]. Proteins were subsequently separated using 10% SDS-polyacrylamide gel electrophoresis, and then were transferred to polyvinylidene difluoride (PVDF) membranes. These membranes were incubated with specific primary antibodies, including mouse anti-COX-2, rabbit anti-iNOS, rabbit anti-IκBα, and rabbit anti-phospho-IκBα antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After an overnight incubation at 4℃, membranes were blocked and incubated with secondary anti-rabbit or anti-mouse IgG-conjugated horseradish peroxidase antibodies (Santa Cruz Biotechnology) for 1-2 h at RT. Immunodetection was performed using an enhanced chemiluminescence western blot detection agent (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Levels of α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were also detected as an internal loading control.
Total RNA was isolated from cultured cells using Trizol (Invitrogen, CA) according to the manufacturer's instructions. cDNA was synthesized by reverse transcription using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). PCR amplification included the following conditions: an initiation step at 94℃ for 5 min, denaturation at 94℃ for 30 s, annealing at 51.5℃ for 45 s, and an extension step at 72℃ for 2 min using Taq polymerase (TAKARA, Tokyo, Japan). The resulting PCR products were separated in a 2% agarose gel containing ethidium bromide. The primers used included: 5'-ATGAGCACAGAAAGCATGATC-3' (forward) and 5'-TACAGGCTTGTCACTCGA ATT-3' (reverse) for TNF-α; 5'-GCCTTCCGTGTTCCTACCC-3' (forward) and 5'-TGCCTGC TTCACCACCTTC-3' (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All samples were normalized to the housekeeping gene, GAPDH, which was not affected by the experimental treatments.
Data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) and GraphPad Prism, version 3.0 (GraphPad Software, Inc., San Diego, CA USA), followed by Tukey's post-hoc test. At least three independent experiments were performed in triplicate. Differences with a P-value less than 0.05 were considered statistically significant.
Various concentrations of SQE (0, 100, 200, and 400 µg/ml), as well as tricin (1 µM) and P-coumaric acid (2.4 µM) at concentrations equivalent to 400 µg/ml SQE, were applied to RAW 264.7 macrophages for 24 h (Fig. 2). Cell viability assays were subsequently performed and the viability detected was greater than 95% for all of the treatment groups. Moreover, the cell viability observed for the SQE, tricin, and P-coumaric acid treated samples was similar to that of the non-treated controls. These results indicate that SQE, tricin, and P-coumaric acid at the concentrations tested are not cytotoxic to RAW 264.7 macrophages.
An in vitro intestinal model was established using a co-culture system of intestinal epithelial Caco-2 cells and RAW 264.7 macrophages (Fig. 1). The Caco-2 and RAW 264.7 macrophages did not contact each other. Therefore, crosstalk between the two cell types was mediated via the secretion of soluble factors by each cell line. Correspondingly, several cytokines involved in gut inflammation were detected following LPS-stimulation to the basolateral side of the model, as a simulation of gut inflammatory process.
It is well-known that macrophages overproduce NO in response to iNOS during the inflammation process [23]. To investigate whether SQE affects the induction of NO in response to LPS in the co-culture model employed for this study, production of NO was detected following the treatment with various concentrations of SQE on the apical side of this model. Following the addition of LPS (1 µg/ml), a two-fold increase in NO levels was detected in the culture medium. In contrast, pretreatment of cells with SQE led to a significant decrease in LPS-induced NO production (Fig. 3A). To further elucidate whether SQE affect other cytokines that contribute to gut inflammation, levels of PGE2, IL-1β, and IL-6 were also analyzed. Similar to NO, LPS-induced production of PGE2, IL-1β, and IL-6 was significantly decreased when cells were pretreated with SQE (Fig. 3B-D)
Tricin and P-coumaric acid are two major bioactive components of SQE [20]. Therefore, the anti-inflammatory effects of tricin and p-coumparic acid were compared with SQE, and the concentrations used for each were analogous to the concentrations of these components in 400 µg/ml SQE. LPS-induced production of NO and IL-6 was inhibited when cells were pretreated with tricin or P-coumaric acid (Fig. 4A, 4D). Pretreatment of cells with tricin or P-coumaric acid also appeared to block LPS-induced PGE2 production, although this inhibition was not statistically significant (Fig. 4B). In contrast, while P-coumaric acid significantly inhibited LPS-induced IL-1β production, tricin only showed an inhibition trend that was not significant (Fig. 4C). SQE exhibited similar efficacy for inhibiting each of the inflammatory mediators assayed to P-coumaric acid, but tricin was less effective (Fig. 4).
Expression of iNOS was detected using western blot in order to determine whether the synthesis of iNOS was affected by NO production. In addition, it is well-known that PGE2 is produced by activated macrophages via induction of COX-2 [24]. Therefore, to identify the potential anti-inflammatory properties of SQE, tricin, and P-coumaric acid, expression of iNOS and COX-2 were analyzed. LPS up-regulated iNOS and COX-2 expression, whereas treatment of RAW 264.7 macrophages with SQE resulted in the down-regulation of LPS-induced expression of both iNOS and COX-2 (Fig. 5A). Moreover, compared to the pretreatment of cells with SQE, tricin, and P-coumaric acid individually, SQE exhibited the highest efficacy for down-regulating expression of iNOS and COX-2, followed by P-coumaric acid (Fig. 5B).
NF-κB is a transcription factor that regulates the production of pro-inflammatory mediators in LPS-stimulated macrophages, and is required for up-regulation of iNOS, COX-2, and TNF-α. LPS-mediated activation of NF-κB is also associated with hyperphosphorylation of IκBα, which induces the subsequent degradation of IκBα [25]. To clarify the mechanism by which SQE and its bioactive compounds, tricin and P-coumaric acid, may inhibit LPS-induced phosphorylation of IκBα and upregulation of TNF-α, LPS-stimulated phosphoryation of IκBα was detected using western blot, and mRNA levels of TNF-α were detected using PCR. LPS treatment resulted in an increase in IκBα phosphorylation and a degradation of IκBα compared with the controls. This increase in phsophorylation and the degradation of IκBα was reduced by treating with SQE and this inhibition was found to be in a dose-dependent (Fig. 6A). However, pretreatment with tricin did not block IκBα phosphorylation, whereas pretreatment with P-coumaric acid did inhibit phosphorylation of IκBα, albeit to a lesser extent that achieved by SQE (Fig. 6B).
When LPS was applied to the basolateral side of the co-culture system, mRNA levels of TNF-α in RAW 264.7 macrophages were also analyzed. Pretreatment with SQE resulted in a downregulation of LPS-induced TNF-α mRNA expression in a dose dependent manner (Fig. 7A). In contrast, tricin did not affect mRNA levels of TNF-α while P-coumaric acid did significantly down-regulate TNF-α levels, but latter levels were lower than those mediated by SQE treatment (Fig. 7B). Taken together, these results are consistent with the western blot data for IκBα phophorylation.
Natural anti-inflammatory agents represent an attractive and relatively safe alternative to the use of toxic drugs for the modulation of inflammatory disorders. Sasa leaves exhibit various beneficial activities, including anti-oxidant, anti-cancer, anti-inflammation, and anti-obesity properties [17,18,19]. However, there is limited evidence to indicate that SQE can attenuate intestinal inflammatory disease. To our knowledge, the present study is the first to identify molecular mechanisms that may be responsible for the anti-inflammatory effects mediated by SQE as well as its bioactive compounds, tricin and P-coumaric acid. In the present study, an in vitro co-culture model was established using epithelial-like Caco-2 cells and murine RAW 264.7 macrophages, and suppression of NF-κB activation and the subsequent induction of inflammatory mediators were observed.
Pro-inflammatory mediators such as NO, TNF-α, PGE2, IL-6, and IL-1β are excessively produced by macrophages during many clinical disorders, including rheumatoid arthritis [26], asthma [27], and IBD [28]. In addition, immune cells of IBD patients have been shown to secret high levels of the pro-inflammatory cytokines, IL-6, IL-1β, and TNF-α [29]. In particular, overexpression of IL-1β and TNF-α have been hypothesized to play a pivotal role in the pathogenesis of IBD [30]. Correspondingly, blocking of these cytokines is proposed to provide an effective treatment strategy for IBD. Indeed, several agents that inhibit the production of IL-1β and TNF-α have been successfully applied in experimental colitis models and human trials [30,31]. In the present study, LPS-induced production of IL-1β and TNF-α was suppressed by SQE and its bioactive compounds, tricin and P-coumaric acid. In IBD, higher levels of IL-1β and TNF-α are accompanied by increase in other pro-inflammatory mediators, including IL-6, iNOS, PGE2, and COX-2. Production of iNOS by macrophages results in the overproduction of NO during the inflammatory process, while synthesis of PGE2 by LPS is due to up-regulation of COX-2, a molecule that mediates various inflammatory processes [32]. Correspondingly, the promoter region of COX-2 contains binding sites for various transcription factors, including NF-κB [33].
NF-κB has been shown to regulate immune and inflammatory signaling, and in some cases represents a rate-limiting step [34]. In general, when cells are activated by inflammatory stimuli, the protein inhibiting NF-κB is degraded. NF-κB is then able to translocate into nucleus to promote the transcription of pro-inflammatory mediators [35]. It has been reported that several natural antioxidants, including carotenoids and polyphenol compounds such as β-carotene, lycopene, and curcumin mediate anti-inflammatory activities by directly suppressing the expression of NF-κB dependent cytokines, including iNOS and COX-2 [35,36]. In the present study, it was demonstrated the SQE inhibits the up-regulation of iNOS and COX-2, and suppresses production of PGE2 in RAW 264.7 macrophages. In addition, SQE inhibited LPS-induced phosphorylation of IκBα. Consequently, activation of NF-κB was suppressed. Since NF-κB is a central player in the signaling cascades mediating inflammation, and is able to simultaneously block the expression of multiple inflammatory mediators, targeting NF-κB appears to be a good strategy for maximizing therapeutic effects. TNF-α also plays an important role in the inflammatory response, and activation of NF-κB has been shown to induce expression of TNF-α [37]. The inhibitory effect of SQE on TNF-α expression was estimated in the present study based on the extent of IκBα phosphorylation that was detected. In future studies, it will be important to investigate whether SQE directly inhibits the DNA-binding of NF-κB p65.
There are numerous studies that have demonstrated the beneficial effects of natural polyphenols on experimental inflammatory dieseases, including ellagic acid and rosemarinic acid [38,39]. Sasa quelpaertensis leaves have traditionally been used as medicine for inflammation mediated diseases, including ulcers and diabetes [17,40]. Correspondingly, SQE has shown to reduce the inflammatory response induced by LPS, specifically by inhibiting the production of NO and the expression of COX-2 and iNOS in RAW 264.7 macrophages [41]. However, to our knowledge, this is the first study investigate the anti-infammatory effects of SQE in an in vivo co-culture model of epithelial interactions in IBD using macrophages. SQE contains various phytochemicals, polysaccharides, amino acids, and polyphenols, including P-coumaric acid and tricin. Moreover, polyphenols have previously been shown to mediate antioxidant and anti-inflammatory effects [42,43]. It has been reported that P-coumaric acid, a common dietary phenolic compound in plants, reduces the oxidation of low-density lipoprotein cholesterol and carbon tetrachloride-induced hepatotoxicity [42,44]. In addition, a polyphenol, tricin has been reported to have an anti-inflammatory role in inflammation-associated colon carcinogenesis [45]. In the present study, P-coumaric acid and tricin exhibited anti-inflammatory effects by regulating the production of pro-inflammatory mediators. However, the regulation provided by SQE was greater than that provided by p-courmaric acid or tricin. Overall, SQE was associated with the greatest reduction in the levels of each LPS-induced pro-inflammatory mediators assayed. This observation is consistent with previous studies which demonstrated that a combination of nutrients can provide synergistic effects [46]. Therefore, the potent anti-inflammatory effects observed for SQE are attributed to the additive or synergistic effects of the phytochemicals and nutrients present in this extract. Based on the results of the present study, further studies are needed to investigate the effect of the bioactive compounds of SQE in an in vivo model of inflammatory-related intestinal disease.
In conclusion, this study demonstrated that SQE and its bioactive compounds, P-coumaric acid and tricin, are able to significantly attenuate pro-inflammatory mediators, including PGE2, IL-6, IL-1β, NO, COX-2, and TNF-α, by increasing the phosphorylation of IκBα in the in vitro co-culture system employed. Furthermore, as a relatively nontoxic natural product, SQE has the potential to treat inflammatory diseases such as IBD. Therefore, further in vivo studies and clinical trials are needed to confirm the results of this study.
Figures and Tables
Fig. 1
Schematic representation of the in vitro co-culture system that was established. Caco-2 cells were grown in 6-well cell culture inserts with a semipermeable support membrane. Murine RAW 264.7 macrophages were cultured in 6-well culture plates. Culture inserts containing Caco-2 cells were placed in the 6-well plates to establish the co-culture system.
Fig. 2
SQE did not affect the viability of RAW 264.7 macrophages in the co-culture system. RAW 264. 7 cells were seeded in 12 well-plates and treated with various doses of SQE (100, 200, and 400 µg/ml), as well as individually with two bioactive compounds, tricin (1 µM) and P-coumaric acid (PC, 2.4 µM), at concentration equivalent to that contained in 400 µg/ml SQE. After 24 h, each set of cells was stained with trypan blue. Both stained and unstained cells were counted, and the proportion of unstained cells was used to calculate cell viability. One-way ANOVA was applied using Tukey's post-hoc test (α = 0.05). SQE, Sasa quelpaertensis extract; PC, P-coumaric acid.
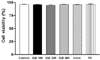
Fig. 3
SQE reduces the expression of inflammatory mediators in the co-culture system of Caco-2 and RAW 264.7 cells. Caco-2 cells were incubated with RAW 264.7 macrophages in the presence of various concentrations of SQE (100, 200, and 400 µg/ml) that were applied to the apical side of this co-culture system. After 3 h, 1 µg/ml LPS was added to the basolateral side and the system was incubated overnight. Levels of NO (A), PGE2 (B), IL-1β (C), and IL-6 (D) were subsequently measured from collected culture medium samples using commercially available ELISA kits. Data were analyzed using one-way ANOVA for multiple comparisons followed by Tukey's post-hoc test (P < 0.05). The different letters are used to indicate significant differences. SQE, Sasa quelpaertensis extract; PC, P-coumaric acid, LPS, lipopolysaccharide.
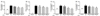
Fig. 4
Tricin and P-coumaric acid suppress the expression of inflammatory mediators in a co-culture system of Caco-2 and RAW 264.7 cells. Tricin (1 µM) and P-coumaric acid (PC, 2.4 µM) were individually added into the apical compartment of a Caco-2/RAW 264.7 co-culture model. After 3 h, 1µg/ml LPS was added to the basolateral compartment and the cells were incubated overnight. Production of NO (A), PGE2 (B), IL-1β (C), and IL-6 (D) were subsequently analyzed from collected media samples using commercially available ELISA kits. Data were analyzed by one-way ANOVA for multiple comparisons followed by Tukey's post-hoc test (P < 0.05). The different letters are used to indicate significant differences. SQE, Sasa quelpaertensis extract; PC, P-coumaric acid, LPS, lipopolysaccharide.
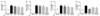
Fig. 5
SQE, tricin, and P-coumaric acid down-regulate the expression of iNOS and COX-2 in a co-culture system of Caco-2 and RAW 264.7 cells. SQE, tricin (1 µM), and P-coumaric acid (PC, 2.4 µM) were individually added into the apical compartment of the Caco-2/RAW 264.7 co-culture model. After 3 h, 1 µg/ml LPS was added to the basolateral compartment and cells were incubated overnight. Expression of iNOS and COX-2 were then analyzed by western blot. (A) Following the addition of various doses of SQE (100, 200, 400 µg/ml), levels of iNOS and COX-2 were analyzed. The representative blots were shown (left panel) and quantified iNOS (middle panel) and COX-2 (right panel) were shown after normalization to α-tubulin. (B) A equivalent dose of tricin or PC based on 400 µg/ml SQE was added, and levels of iNOS and COX-2 were analyzed. The representative blot has shown (left panel). The quantified iNOS (middle panel) and COX-2 (right panel) were shown after normalization to α-tubulin. One-way ANOVA was applied using Tukey's post-hoc test (α = 0.05). The different letters indicate significant differences. SQE, Sasa quelpaertensis extract; PC, P-coumaric acid, LPS, lipopolysaccharide.

Fig. 6
SQE, P-coumaric acid, and tricin decrease IκBα phosphorylation. SQE, tricin (1 µM), and P-coumaric acid (PC, 2.4 µM) acid were individually added into the apical compartment of the Caco-2/RAW 264.7 co-culture model. After 3 h, 1 µg/ml LPS was added to the basolateral compartment and the cells were incubated overnight. Phosphorylation of IκBα was detected by western blot. (A) Following the addition of various doses of SQE (100, 200, 400 µg/ml), levels of phospho-IκBα and IκBα were analyzed. The representative blots were shown (left panel) and quantified phospho-IκBα (middle panel) and IκBα (right panel) were presented with normalization to α-tubulin. (B) A equivalent dose of tricin or P-coumaric acid based on 400 µg/ml SQE was added, and the levels of phospho-IκBα and IκBα were analyzed. The representative blots were shown (left panel) and quantified phospho-IκBα (middle panel) and IκBα (right panel) were presented after normalization to α-tubulin. One-way ANOVA was applied using Tukey's post-hoc test (α = 0.05). The different letters indicate significant differences. SQE, Sasa quelpaertensis extract; PC, P-coumaric acid, LPS, lipopolysaccharide.

Fig. 7
SQE, P-coumaric acid, and tricin down-regulate mRNA levels of TNF-α. SQE, tricin (1 µM), and P-coumaric acid (PC, 2.4 µM) were individually added into the apical compartment of the Caco-2/RAW 264.7 co-culture model. After 3 h, 1 µg/ml LPS was added to the basolateral compartment and cells were incubated overnight. TNF-α mRNA expression was analyzed using PCR. (A) Following the addition of various doses of SQE (100, 200, 400 µg/ml), levels of TNF-α mRNA were detected. The representative blots were shown (upper panel) and quantified TNF-α expression with normalization to GAPDH was presented (lower panel). (B) An equivalent dose of tricin or P-coumaric acid based on 400 µg/ml SQE was added and mRNA levels of TNF-α were detected. The representative blots has shown (upper panel) and quantified TNF-α expression with normalization to GAPDH was presented (lower panel). One-way ANOVA was applied using Tukey's post-hoc test (α = 0.05). The different letters indicate significant differences. SQE, Sasa quelpaertensis extract; PC, P-coumaric acid, LPS, lipopolysaccharide, GAPDH, glyceraldehydes-3-phosphate dehydrogenase.

References
1. Arai F, Takahashi T, Furukawa K, Matsushima K, Asakura H. Mucosal expression of interleukin-6 and interleukin-8 messenger RNA in ulcerative colitis and in Crohn's disease. Dig Dis Sci. 1998; 43:2071–2079.
2. Chung HL, Yue GG, To KF, Su YL, Huang Y, Ko WH. Effect of Scutellariae Radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World J Gastroenterol. 2007; 13:5605–5611.

3. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002; 31:1–20.

4. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533.

5. Menditto A, Menotti A, Morisi G, Patriarca M, Spagnolo A. Serum ascorbic acid levels in men aged 55-75 years: association to selected social factors and biochemical parameters. Arch Gerontol Geriatr. 1992; 15:Suppl 1. 257–265.

6. Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T, Mizuno M. Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol. 2009; 9:1444–1451.

7. Bode H, Schmitz H, Fromm M, Scholz P, Riecken EO, Schulzke JD. IL-1beta and TNF-alpha, but not IFN-alpha, IFN-gamma, IL-6 or IL-8, are secretory mediators in human distal colon. Cytokine. 1998; 10:457–465.

8. Brozek W, Bises G, Fabjani G, Cross HS, Peterlik M. Clone-specific expression, transcriptional regulation, and action of interleukin-6 in human colon carcinoma cells. BMC Cancer. 2008; 8:13.

9. Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009; 121:1–13.

10. Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci (Lond). 2009; 116:451–465.

11. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990; 98:694–702.

12. Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993; 75:253–261.

13. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993; 75:263–274.

14. Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, Miyazaki J, Kiyono H. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta + NK1.1 + T cells for the development of small intestinal inflammation. J Immunol. 2002; 169:460–468.

15. Farombi EO, Adedara IA, Ajayi BO, Ayepola OR, Egbeme EE. Kolaviron, a natural antioxidant and anti-inflammatory phytochemical prevents dextran sulphate sodium-induced colitis in rats. Basic Clin Pharmacol Toxicol. 2013; 113:49–55.

16. Okabe S, Takeuchi K, Takagi K, Shibata M. Stimulatory effect of the water extract of bamboo grass (Folin solution) on gastric acid secretion in pylorus-ligated rats. Jpn J Pharmacol. 1975; 25:608–609.

17. Choi YJ, Lim HS, Choi JS, Shin SY, Bae JY, Kang SW, Kang IJ, Kang YH. Blockade of chronic high glucose-induced endothelial apoptosis by Sasa borealis bamboo extract. Exp Biol Med (Maywood). 2008; 233:580–591.

18. Ren M, Reilly RT, Sacchi N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004; 24:2879–2884.
19. Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Ko HC, Kim SJ. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci Biotechnol Biochem. 2012; 76:755–761.

20. Kim K, Lim JY, Min S, Lim Y, Ko H, Kim S, Kim Y. Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium (DSS)-induced colitis im mice. Nutr Res. Forthcoming 2014.
21. Tanoue T, Nishitani Y, Kanazawa K, Hashimoto T, Mizuno M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem Biophys Res Commun. 2008; 374:565–569.

22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976; 72:248–254.

23. Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996; 111:1524–1533.

24. Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005; 76:608–613.

25. Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996; 64:825–828.

26. Jeoung BR, Lee KD, Na CS, Kim YE, Kim B, Kim YR. Ganghwaljetongyeum, an anti-arthritic remedy, attenuates synoviocyte proliferation and reduces the production of proinflammatory mediators in macrophages: the therapeutic effect of GHJTY on rheumatoid arthritis. BMC Complement Altern Med. 2013; 13:47.

27. Lee H, Bae S, Choi BW, Yoon Y. WNT/beta-catenin pathway is modulated in asthma patients and LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol Immunotoxicol. 2012; 34:56–65.

28. Okada Y, Tsuzuki Y, Narimatsu K, Sato H, Ueda T, Hozumi H, Sato S, Hokari R, Kurihara C, Komoto S, Watanabe C, Tomita K, Kawaguchi A, Nagao S, Miura S. 1,4-Dihydroxy-2-naphthoic acid from Propionibacterium freudenreichii reduces inflammation in interleukin-10-deficient mice with colitis by suppressing macrophage-derived proinflammatory cytokines. J Leukoc Biol. 2013; 94:473–480.

29. MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999; 19:266–272.
30. Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003; 9:1107–1113.

31. Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, Bala M, Johanns J, Olson A, Hanauer SB. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007; 102:794–802.

32. Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003; 17:1411–1421.

33. Appleby SB, Ristimäki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994; 302(Pt 3):723–727.

34. Beg AA, Finco TS, Nantermet PV, Baldwin AS Jr. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993; 13:3301–3310.

35. Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150:43–56.

36. Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kwon YG, Chung CK, Kim YM. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med. 2005; 37:323–334.

37. Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000; 164:4277–4285.

38. Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn's disease. Biochem Pharmacol. 2011; 82:737–745.

39. Chu X, Ci X, He J, Jiang L, Wei M, Cao Q, Guan M, Xie X, Deng X, He J. Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules. 2012; 17:3586–3598.

40. Otani K, Yanaura S, Yuda Y, Kawaoto H, Kajita T, Hirano F, Osawa F, Inouye S. Histo-chemical studies on the anti-ulcer effect of bamboo grass in rats. Int J Tissue React. 1990; 12:319–332.
41. Moon JY, Yang EJ, Kim SS, Kang JY, Kim GO, Lee NH, Hyun CG. Sasa quelpaertensis phenylpropanoid derivative suppresses lipopolysaccharide-induced nitric oxide synthase and cyclo-oxygenase-2 expressions in RAW 264.7 cells. Yakugaku Zasshi. 2011; 131:961–967.

42. Zang LY, Cosma G, Gardner H, Shi X, Castranova V, Vallyathan V. Effect of antioxidant protection by P-coumaric acid on low-density lipoprotein cholesterol oxidation. Am J Physiol Cell Physiol. 2000; 279:C954–C960.
43. Pragasam SJ, Venkatesan V, Rasool M. Immunomodulatory and anti-inflammatory effect of P-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013; 36:169–176.

44. Pérez-Alvarez V, Bobadilla RA, Muriel P. Structure-hepatoprotective activity relationship of 3,4-dihydroxycinnamic acid (caffeic acid) derivatives. J Appl Toxicol. 2001; 21:527–531.

45. Shalini V, Bhaskar S, Kumar KS, Mohanlal S, Jayalekshmy A, Helen A. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: possible role in the inflammatory signaling. Int Immunopharmacol. 2012; 14:32–38.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download