Abstract
The purpose of the study was to assess the effects of a 12 weeks aged garlic extract (AGE) regimen with regular exercise on cardiovascular disease (CVD) risk in postmenopausal women. A total of 30 postmenopausal women (54.4 ± 5.4 years) were randomly divided into the following four groups: Placebo (Placebo; n = 6), AGE intake (AGEI; n = 8), exercise and placebo (Ex + Placebo; n = 8), exercise and AGE (Ex + AGE; n = 8) groups. The AGE group consume 80 mg per day, and exercise groups performed moderate exercise (aerobic and resistance) three times per week. After 12 weeks of treatment, body composition, lipid profile, and CVD risk factors were analyzed. Body weight was significantly decreased in AGEI, Ex + Placebo, and Ex + AGE groups compared to baseline. Body fat % was significantly decreased in the AGEI and Ex + Placebo groups. Body mass index (BMI) was significantly decreased in the AGEI, Ex + Placebo, and Ex + AGE groups. Fat-free mass was significantly decreased in the AGEI group. Total cholesterol (TC) was significantly lower in the Ex + Placebo compared to the Placebo group. AGE supplementation or exercise effectively reduced low-density lipoprotein (LDL-C). Triglyceride (TG) was significantly increased in the AGEI group. Malondialdehyde (MDA) levels were significantly decreased in the AGEI, Ex + Placebo, and Ex + AGE compared to the placebo group. AGE supplementation reduced homocysteine levels regardless of whether the women also exercised. The present results suggest that AGE supplementation reduces cardiovascular risk factors independently of exercise in postmenopausal women.
Menopause is a well-known risk factors for cardiovascular disease (CVD) and leads to increased metabolic parameters [1,2]. The evidence shows that changes in the reproductive hormones affect vascular function in postmenopausal women [3,4]. Other CVD risk factors include excessive weight, diabetes mellitus, dyslipidemia, hypertension, endothelial dysfunction and physical inactivity [5-9], which are associated with impaired cardiac function, dysregulated lipid metabolism, and increased oxidative stress level [10-12].
A primary preventative measure for postmenopausal women with increased CVD risk factors is exercise training with or without diet interventions [13,14]. Duvernoy et al. [15] and Yoshizawa et al. [16] demonstrated that regular exercise significantly improved physical fitness and attenuated CVD risk factors in postmenopausal women. Moreover, previous studies have demonstrated that regular exercise training enhances endothelial function, which has profound stimulatory effects on key vasodilatory enzymes, such as nitrite/nitrate, endothelin-1, and homocysteine [17-19].
Several evidence suggests that nutrition is very important for decreasing CVD risk factors in postmenopausal women, and phytochemical agents found in herbs and plants can be beneficial effect [20-22]. Garlic has been used to treat hypertension, coronary diseases, and CVD, in adult since ancient times [23,24]. Aged garlic extract (AGE) is an odorless product that is made from garlic that has been aged at room temperature. It is bioavailable in animals and humans following oral administration [23-25]. Previous studies have demonstrated that AGE reduces lipid cholesterol, oxidative stress, blood pressure, and improves endothelial function [26-30]. However, the basic mechanisms behind these positive effects are not fully understood in postmenopausal women, and the effects of combined intervention with exercise are largely unknown. Accordingly, we hypothesized that combined intervention may reduce CVD risks to a greater extent than mono-therapy with either treatment.
Therefore, we evaluated the effects of a 12 week AGE regimen with or without regular exercise on body composition, lipid profile, and CVD risk factors in postmenopausal women.
Women were eligible if they had experienced menopause, which was defined as the absence of menstruation for at least 1 year, and had not received hormone therapy (HT) for the past 6 months. Study participants were recruited from the general population through advertisements and the local public health center. Exclusion criteria included CVD, pulmonary, or metabolic disease (hypertension, dyslipidemia, diabetes, etc.), orthopedic problems, and smoking. Before participation, all subjects provided written informed consent on an acknowledgment form approved by the institutional human research committee.
A total 33 of apparently healthy, sedentary postmenopausal women participated in this study. Three participants were excluded due to a diagnosis of hypertension. Subjects (n = 30, age: 54 ± 5.4, body weight: 60.2 ± 6.7 kg, body mass index (BMI): 24.0 ± 2.4) were randomly divided into the following interventions: placebo group (Placebo; n = 6), AGE intake group (AGEI; n = 8), exercise and placebo group (Ex + Placebo; n = 8), exercise and AGE group (Ex + AGE; n = 8).
Each subject reported to the laboratory at 9:00 a.m. following an overnight fast on two separate occasions (pretraining, posttraining) for blood sampling. Participants were asked not to consume caffeine or alcohol for at least 12 hours and not to perform exercise training for 48 hours before the measurement. Participants rested for a minimum of 10 minutes before their blood pressures was measured over brachial artery using a semiautomated device (VP-1000; Colin Medical Technology, Komaki, Japan) while they were in a supine position.
The Korean Garlic Corporation (Ulsung, Korea), provided bioavailable standardized AGE supplements. AGE was provided in packs containing 80 ml of AGE, which was derived from organically grown garlic, and also contained sulfide, disulfide, S-allycysteine (SAC), and protein. Concentrated AGE was further diluted to 65 brix with distilled water. Placebo packs were matched to the active pack with regard to shape. AGE and placebo intake groups consumed one pack five times a week for 12 weeks.
Exercise training groups trained three times per week on nonconsecutive days during the study period. The training program consisted of two mixed activities (aerobic and resistance) as previously described [31]. The exercise intensities for resistance and aerobic training were adjusted to keep the heart rate (HR) at 60% of the predicted maximum HR throughout the study. Exercise training was supervised by a professional educator. Exercise HRs was measured using a polar device (Electro; Oy, Kempele, Finland).
Blood was collected in vacutainer (BD Bioscience, San Jose, CA, USA) from the antecubital vein of the arm following an overnight fast. After centrifugation at 1500 × g for 15 min, separated serum and plasma were stored in multiple aliquots at -80℃ until they were assayed. After completion of the protocol, samples from each subject were analyzed in the same group to minimize laboratory variability.
Body composition, blood pressure and blood sample were evaluated before and 12 weeks. Body composition was measured with a pristine analyzer that uses multi frequency electric impedance to assess weight, fat percentage, and fat-free mass (Jawon Medical, Plus 720). BMI was calculated using the equation of weight (kg)/height (m2).
Systolic and diastolic BP (SBP and DBP, respectively) were measured using a semiautomated device (VP-1000; Colin Medical Technology, Komaki, Japan) with subjects in the supine position. BP was assessed twice, and then the average of the two values was used for subsequent analyses. Mean arterial pressure (MAP) was calculated as MAP = (1/3) SBP + (2/3) DBP; pulse pressure (PP) was calculated as PP = SBP - DBP.
Fasting blood samples were taken from antecubital vein. Serum total cholesterol (TC), triglyceride (TG), and high-density (HDL-C) and low-density (LDL-C) lipoprotein levels were measured using enzymatic techniques based on colorimetric measurement with Bayer kits (ADVIA 1650, Japan). The plasma level of malondialdehyde (MDA; Oxis, USA), endothelin-1 (ET-1; R & D Systems, USA) was measured using an enzyme-linked immunosorbent assay (ELISA) method. Nitrite/nitrate (R & D Systems, USA) were measured using an enzyme immunoassay (EIA) method. Insulin (Roche, Germany) was measured using an enhanced chemiluminescence assay (ECLIA) method. Homocysteine (ABBOTT, USA) was measured using a carbonylmetalloimmunoassay (CMIA) method. All assays were performed according to manufacturers' instructions.
To determine the effect of each intervention on all data, repeated-measures analysis of variance (ANOVA) was used. When indicated by a significant main effect or interaction, specific mean comparisons were performed to identify significant differences within each intervention. In the case of a significant F value, a post hoc test using Tukey's least significant difference (LSD) method was used to identify significant differences among mean values. All data were reported as means ± standard deviation (SD). Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS, version 19.0 (SPSS, Chicago, IL).
The study included 30 Korean women 54.4 ± 5.4 years of age who were classified as postmenopausal and had not had menstrual cycle for at least 1 year. There were no differences in baseline parameters between the groups. Participant characteristics at baseline and after AGE intervention are presented in Table 1.
Following exercise and AGE intervention, body weight was significantly decreased in the AGE, Ex + Placebo (P < 0.001), and Ex + AGE (P < 0.01) groups. Body fat % was significantly decreased in AGE (P < 0.001), and Ex + Placebo (P < 0.01) groups. BMI was significantly decreased in AGEI (P < 0.001), Ex + Placebo (P < 0.01), and Ex+AGE (P < 0.5) groups. Fat-free mass was significantly decreased in the AGEI group (P < 0.01). However, there were no statistically significant body composition changes among the groups.
After 12 week, TC was significantly lower in the Ex + Placebo group compared the Placebo group (P < 0.01). TC levels slightly decreased with intervention, but the difference was not statistically significant. LDL was significantly lower in the AGEI, Ex + Placebo, and Ex + AGE groups compared to the Placebo group (P < 0.01). MDA level was significantly lower in AGEI, Ex + Placebo (P < 0.01), and Ex + AGE groups (P < 0.05), Ex + Placebo group was significantly lower than among the groups. (P < 0.05) (Fig.1). HDL-C was not different among the groups. TG was significantly increased in the Placebo group, but AGE or exercise suppressed this increase (Table 2).
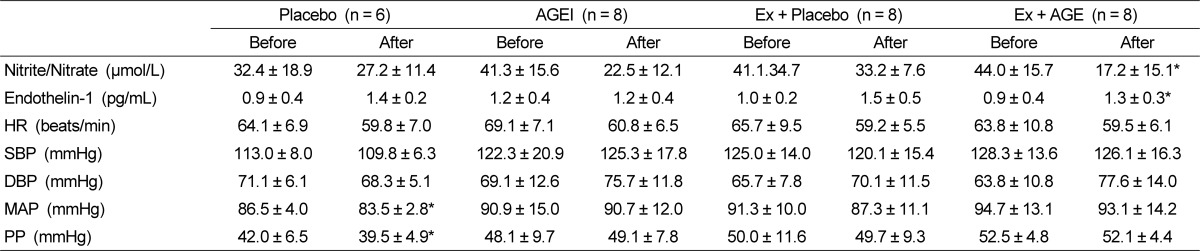
Homocysteine was significantly decreased with intervention in AGEI, and Ex + AGE (P < 0.05) (Fig.1). Nitrate/nitrite level was significantly decreased in the Ex + AGE intervention (P < 0.05). However, ET-1 was significantly increased in the Ex + AGE group (P < 0.05). There were no significantly differences between baseline and intervention in blood pressure, HR, SBP, or DBP at rest. MAP was slightly decreased in the Ex + Placebo group, however, it was not statistically significant MAP and PP were significantly decreased in the Placebo group (P < 0.05). These were no statistically significant changes among the groups (Table 3).
The purpose of this study was to investigate the effects of a 12-weeks AGE regimen with regular exercise on CVD risk factors in healthy postmenopausal women by measuring body composition, lipid profiles, an oxidative stress marker, and vascular function. The major finding of the this study demonstrate that AGE supplementation and/or exercise training results in beneficial effects on body weight, BMI, LDL-C, MDA, and homocysteine, which are important CVD risk factors.
Menopause increases the risk of CVD because it affects metabolic parameters and endothelial dysfunction. These changes include increased fat mass, cholesterol level, and oxidative stress and, decreased vasodilatations, all of which can be controlled by exercise, nutrition, or a combination of the two treatment [16,22,32]. AGE is safe, and previous studies have demonstrated that it is more effective than garlic oil, powder, and raw garlic in relation to decreasing CVD risk factors [33,34]. In addition, AGE contains SAC, which is more easily absorbed than garlic power [35].
In the present study, the effect of AGE supplementation is primarily shown by decreased body weight and fat. Regular AGE supplementation significantly decreased body weight and fat compared to the Placebo group, and a similar effect was achieved with a 12-week exercise regimen. Although these findings demonstrate that AGE supplementation alone may inhibit increasing fat mass, we did not determine the mechanism. Furthermore, the combination of AGE and exercise synergistically promoted greater body weight loss than either treatment alone. Because obesity with high fat accumulation is a major risk factor for CVD, and weight loss decreases CVD risk, these results suggest that AGE intake potentially decreased CVD risk in postmenopausal women.
Many researchers have suggested that lifestyle modifications such as exercise and dietary changes were recommended as preventative measures for postmenopausal women [36-38]. In our study, 12 weeks of regular exercise training decreased body weight, LDL-C, and MDA in postmenopausal women. In agreement our results, Figueroa et al. [31] demonstrated that exercise training improves metabolic parameters in postmenopausal women. However, we hypothesized that AGE supplementation and exercise training would decrease CVD risk factors to a greater degree than either treatment alone. However, we did not observe an addictive effect. These results indicate that the effects of AGE intake and regular exercise training may be independent. Importantly, deceased CVD risk may be achieved by AGE supplementation alone in postmenopausal women.
Nitrosative stress occurs when excessive reactive nitrogen species production in a system exceeds the organism's ability to neutralize and remove them. Nitric (NO) overproduction may alter protein structure via nitrosylation or lead to lipid peroxidation [39]. In our study, AGE supplement significantly reduced formation of nitrite/nitrite levels which is the end-product of NO.
Elevated plasma homocysteine is a risk factor for CVD that is independent of hypercholesterolemia [40]. Although there is controversy regarding the mechanism underlying homocysteine-induced CVD, there is a clear relationship between plasma homocysteine level and CVD risk; therefore, lowering plasma homocysteine is beneficial for reducing the incidence of CVD [41]. In agreement with animal experiments [42], AGE supplementation in postmenopausal women lowered plasma homocysteine, but exercise alone did not. We did not observe an additive effect in the group that received AGE and exercised. Our results indicate that AGE supplementation may be useful for postmenopausal women with elevated homocysteine levels.
It is well-known that ET-1 induces strong vasoconstriction activity in vascular smooth muscle cells [43]. Increased circulation levels of ET-1 in postmenopausal women have been previously reported [44]. In this study, we found that plasma ET-1 concentrations were only significantly increased in the Ex + AGE group. It is not clear why this group showed a significantly higher level of ET-1, but it may be related to the aging process or unidentified physiological change during menopause following AGE supplementation or exercise because the mean ET-1 value among the groups was similar.
The present study has several limitations. The sample size was small; eight participants in each group may not have been sufficient to obtain statistically significant results. A further study with a larger number of participants is necessary. Furthermore, a longer study period may yield more significant results.
In conclusion, we demonstrated that 12 weeks of AGE supplementation might provide independent benefits on reducing CVD risk factors for post menopausal women without hindering the well known beneficial effect of exercise.
References
1. Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J Am Coll Cardiol. 2009; 54:2374–2375. PMID: 20082926.
2. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987; 316:1105–1110. PMID: 3574358.

3. Utian WH. Biosynthesis and physiologic effects of estrogen and pathophysiologic effects of estrogen deficiency: a review. Am J Obstet Gynecol. 1989; 161:1828–1831. PMID: 2690634.

4. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005; 308:1583–1587. PMID: 15947175.

5. Andreassi MG, Barale R, Iozzo P, Picano E. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis. 2011; 26:77–83. PMID: 21164186.

6. Gijón-Conde T, Banegas JR. Cardiovascular disease in hypertension: gender differences in 100,000 clinical records. Rev Clin Esp. 2012; 212:55–62. PMID: 21917249.
7. Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther. 2006; 111:384–399. PMID: 16343639.

8. Purushothaman KR, Meerarani P, Moreno PR. Inflammation and neovascularization in diabetic atherosclerosis. Indian J Exp Biol. 2007; 45:93–102. PMID: 17249333.
9. Kruger HS, Venter CS, Vorster HH. THUSA Study. Physical inactivity as a risk factor for cardiovascular disease in communities undergoing rural to urban transition: the THUSA study. Cardiovasc J S Afr. 2003; 14:16–23. PMID: 12621539.
10. Armstrong K, Rakhit D, Jeffriess L, Johnson D, Leano R, Prins J, Garske L, Marwick T, Isbel N. Cardiorespiratory fitness is related to physical inactivity, metabolic risk factors, and atherosclerotic burden in glucose-intolerant renal transplant recipients. Clin J Am Soc Nephrol. 2006; 1:1275–1283. PMID: 17699359.

11. Laufs U, Wassmann S, Czech T, Münzel T, Eisenhauer M, Böhm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005; 25:809–814. PMID: 15692095.

12. Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J. Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality--16 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord. 2000; 24:1465–1474. PMID: 11126344.
13. Riesco E, Aubertin-Leheudre M, Maltais ML, Audet M, Dionne IJ. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women: a pilot study. Menopause. 2010; 17:1035–1039. PMID: 20539245.
14. Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009; 297:H1899–H1903. PMID: 19783777.

15. Duvernoy CS, Martin JW, Briesmiester K, Muzik O, Mosca L. Self-reported physical activity and myocardial flow reserve in postmenopausal women at risk for cardiovascular disease. J Womens Health (Larchmt). 2006; 15:45–50. PMID: 16417417.

16. Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens. 2010; 23:368–372. PMID: 20075849.

17. Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005; 569:913–924. PMID: 16223762.
18. Okura T, Rankinen T, Gagnon J, Lussier-Cacan S, Davignon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Effect of regular exercise on homocysteine concentrations: the HERITAGE Family Study. Eur J Appl Physiol. 2006; 98:394–401. PMID: 17016702.

19. Brett SE, Jiang BY, Turner C, Ritter JM, Chowienczyk PJ. Elevation of plasma homocysteine by methionine loading increases the diastolic blood pressure response to exercise. J Hypertens. 2006; 24:1985–1989. PMID: 16957558.

20. Squadrito F, Altavilla D, Morabito N, Crisafulli A, D'Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002; 163:339–347. PMID: 12052481.

21. Liu ZM, Ho SC, Chen YM, Ho YP. The effects of isoflavones combined with soy protein on lipid profiles, C-reactive protein and cardiovascular risk among postmenopausal Chinese women. Nutr Metab Cardiovasc Dis. 2011; Epub ahead of print.

22. Kim SY, Seo SK, Choi YM, Jeon YE, Lim KJ, Cho S, Choi YS, Lee BS. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: a double-blind randomized controlled trial. Menopause. 2012; 19:461–466. PMID: 22027944.
23. Sobenin IA, Andrianova IV, Fomchenkov IV, Gorchakova TV, Orekhov AN. Time-released garlic powder tablets lower systolic and diastolic blood pressure in men with mild and moderate arterial hypertension. Hypertens Res. 2009; 32:433–437. PMID: 19390538.

24. Capraz M, Dilek M, Akpolat T. Garlic, hypertension and patient education. Int J Cardiol. 2007; 121:130–131. PMID: 17088002.

25. Morihara N, Sumioka I, Ide N, Moriguchi T, Uda N, Kyo E. Aged garlic extract maintains cardiovascular homeostasis in mice and rats. J Nutr. 2006; 136:777S–781S. PMID: 16484562.

26. Morihara N, Hayama M, Fujii H. Aged garlic extract scavenges superoxide radicals. Plant Foods Hum Nutr. 2011; 66:17–21. PMID: 21318303.

27. Ried K, Frank OR, Stocks NP. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas. 2010; 67:144–150. PMID: 20594781.

28. Morihara N, Ide N, Weiss N. Aged garlic extract inhibits homocysteine-induced scavenger receptor CD36 expression and oxidized low-density lipoprotein cholesterol uptake in human macrophages in vitro. J Ethnopharmacol. 2011; 134:711–716. PMID: 21256950.
29. Morihara N, Sumioka I, Moriguchi T, Uda N, Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002; 71:509–517. PMID: 12052435.

30. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009; 3:156–161. PMID: 20016716.

31. Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011; 18:980–984. PMID: 21540753.

32. Pereira IR, Faludi AA, Aldrighi JM, Bertolami MC, Saleh MH, Silva RA, Nakamura Y, Campos MF, Novaes N, Abdalla DS. Effects of soy germ isoflavones and hormone therapy on nitric oxide derivatives, low-density lipoprotein oxidation, and vascular reactivity in hypercholesterolemic postmenopausal women. Menopause. 2006; 13:942–950. PMID: 17019381.

33. Harauma A, Moriguchi T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J Nutr. 2006; 136:769S–773S. PMID: 16484560.

34. Hoshino T, Kashimoto N, Kasuga S. Effects of garlic preparations on the gastrointestinal mucosa. J Nutr. 2001; 131:1109S–1113S. PMID: 11238827.

35. Lawson LD, Gardner CD. Composition, stability, and bioavailability of garlic products used in a clinical trial. J Agric Food Chem. 2005; 53:6254–6261. PMID: 16076102.

36. Imayama I, Alfano CM, Kong A, Foster-Schubert KE, Bain CE, Xiao L, Duggan C, Wang CY, Campbell KL, Blackburn GL, McTiernan A. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011; 8:118. PMID: 22026966.

37. Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr. 2002; 132:930–935. PMID: 11983816.

38. Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003; 27:1066–1071. PMID: 12917712.

39. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84. PMID: 16978905.

40. Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991; 324:1149–1155. PMID: 2011158.

41. Moat SJ, Lang D, McDowell IF, Clarke ZL, Madhavan AK, Lewis MJ, Goodfellow J. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004; 15:64–79. PMID: 14972346.

42. Yeh YY, Yeh SM. Homocysteine-lowering action is another potential cardiovascular protective factor of aged garlic extract. J Nutr. 2006; 136:745S–749S. PMID: 16484555.

43. Miyauchi A. Pathophysiology and treatment of malignancyassociated hypercalcemia. Nihon Rinsho. 2006; (Suppl 2):161–164. PMID: 16817374.
44. Cagnacci A, Tarquini R, Perfetto F, Arangino S, Zanni AL, Cagnacci P, Facchinetti F, Volpe A. Endothelin-1 and nitric oxide levels are related to cardiovascular risk factors but are not modified by estradiol replacement in healthy postmenopausal women. A cross-sectional and a randomized cross-over study. Maturitas. 2003; 44:117–124. PMID: 12590007.

Fig. 1
Effect of exercise and AGE intervention on blood markers in postmenopausal women. TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; MDA, malondialdehyde. #P < 0.05 vs placebo. ##P < 0.01 vs placebo. *P < 0.05 vs before intervention. **P < 0.01 vs before intervention.
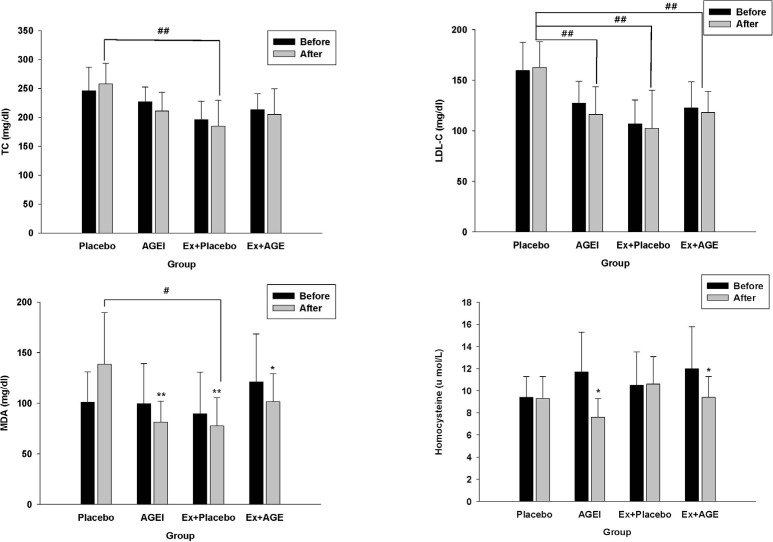
Table 1
Participant characteristics at baseline and after the exercise and AGE intervention in postmenopausal women1)
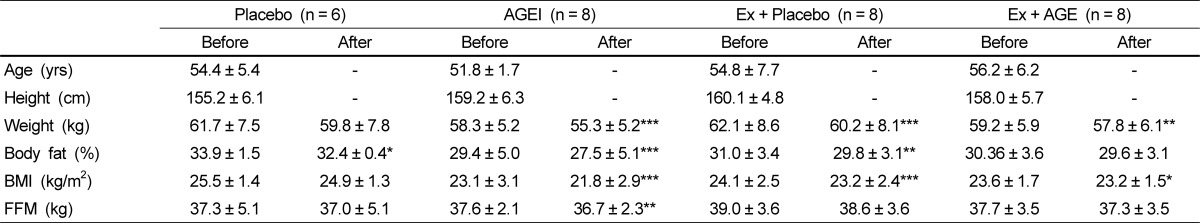
Table 2
Effect of exercise and AGE intervention on markers of lipid metabolism in postmenopausal women1)





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download