Abstract
Eotaxin is an important inflammatory chemokine in eosinophil chemotaxis and activation and, thus, is implicated in asthma. Recently, obesity was associated with an increased prevalence of asthma, but the relationship between obesity and eotaxin expression has only been partially understood in obese mice and human studies. Therefore, we studied the expression patterns of eotaxin in 3T3-L1 preadipocytes/adipocytes to determine whether eotaxin levels are influenced by body weight gain and/or reduction in diet-induced obese mice. First, we investigated eotaxin expression during differentiation in 3T3-L1 adipocytes. Then, we treated 3T3-L1 preadipocytes/adipocytes with tumor necrosis factor-alpha (TNF-α), eotaxin, interleukin (IL)-4, IL-5, or leptin. To examine the effects of weight loss in high-fat diet induced obese mice, we fed C57BL/6 mice a high-fat diet or a normal diet for 26 weeks. Then, half of the high-fat diet group were fed a normal diet until 30 weeks to reduce weight. Epididymal adipose tissue, visceral adipose tissue, serum, and bronchoalveolar fluid of mice were examined for eotaxin expression. The results showed that eotaxin expression levels increased with adipocyte differentiation and that more eotaxin was expressed when the cells were stimulated with TNF-α, eotaxin, IL-4, IL-5, or leptin. An in vivo study showed that eotaxin levels were reduced in visceral adipose tissues when high-fat diet fed mice underwent weight loss. Taken together, these results indicate a close relationship between eotaxin expression and obesity as well as weight loss, thus, they indirectly show a relation to asthma.
Eotaxin (CCL11), an inflammatory chemokine, is an eosinophil-specific chemoattractant that induces infiltration of eosinophils to the lung. Moreover, it is the predominant substance in eosinophilia [1,2]. Human eotaxin is a non-glycosylated polypeptide comprised of 74-amino-acid residues with a molecular weight of 8.3-kDa [3]. Eotaxin is secreted by epithelial cells, fibroblasts, macrophages, and eosinophils [4-6].
In human studies, obese individuals have higher amounts of adipose tissue and serum eotaxin levels than lean individuals. In particular, visceral fat secretes more eotaxin than subcutaneous adipose tissue [7]. In 2006, a study of the correlation between weight and serum eotaxin levels showed that of 79 types of proteins, including growth factors, cytokines, and chemokines, the levels of monocyte chemoattractant protein-4 and eotaxin were higher in obese individuals than lean individuals, according to an assessment of serum in eight lean individuals and seven obese individuals with antibody-based protein microarray [8]. This result shows that eotaxin and obesity have a close relationship in humans.
Many clinical studies have reported that obesity is associated with the incidence and severity of asthma [9-11], and that increases in body mass index (BMI) affect asthma symptoms, airway hyper-responsiveness, and atopy [12,13]. However, clear correlations have not been established.
A relationship between obesity and asthma was clearly shown when obese patients with asthma showed reduced symptoms of asthma and reduced serum eotaxin levels during weight loss attempts [14,15]. However, this only revealed the phenomenon, and more research on the mechanism is needed.
In human adipocytes (SGBS), assessed by DNA microarray, the eotaxin gene was expressed and its level increased with tumor necrosis factor-alpha (TNF-α) treatment [16]. However, according to the research released by other groups in 2007, eotaxin mRNA expression in 3T3-L1 preadipocytes is more than that of differentiated adipocytes, and increases with lipopolysaccharide (LPS) stimulation [17]. Moreover, stroma vascular cells, including macrophages, preadipocytes, and eosinophils that infiltrated adipose tissue, secrete more eotaxin than adipocytes in adipose tissue [7]. These results show the necessity for in-depth research on eotaxin secretion in differentiated adipocytes and eotaxin secretion in adipocytes due to interaction with infiltrates.
A study modeled on diet-induced obese mice showed that although obese mice had higher eotaxin levels in bronchoalveolar lavage fluid (BALF), serum eotaxin levels were similar to the control [18]. Conversely, other studies have shown that obese mice have 2-fold higher serum eotaxin levels than controls [7]. These contradictory results on eotaxin levels in serum and BALF, again, mandate research on the change in eotaxin level due to obesity. In this study, we examined eotaxin expression in 3T3-L1 adipocytes and examined the effects of obesity and weight loss by investigating the change in eotaxin levels in BALF, serum, and adipose tissues of mice.
High-glucose DMEM, bovine calf serum (BCS), and fetal bovine serum (FBS) were purchased from Hyclone (Logan, Utah, USA). Trypsin/EDTA, insulin, dexamethasone (dex), 3-isobutyl-1-methylxanthine (IBM-X) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Trizol for RNA extraction and ImProm II reverse transcriptase were purchased from Promega (Madison, WI, USA). 2 × SYBR green mix was purchased from USB (Cleveland, OH, USA). All primers and oligo dT primers were purchased from Bioneer (Daejeon, Korea).
Mouse eotaxin ELISA kits were purchased from R & D Systems (Minneapolis, MN, USA). Recombinant mouse TNF-α was purchased from Sigma-Aldrich. Recombinant mouse eotaxin, IL-4, IL-5, and leptin were purchased from PromoKine (Heidelberg, Germany).
Mouse 3T3-L1 pre-adipocytes were grown to confluence in DMEM with 10% BCS at 37℃ in a humidified atmosphere of 5% CO2. At 1 day post-confluence (designated day 0), cell differentiation was induced with a mixture of IBMX (0.5 mM), dex (0.25 mM), and insulin (1 mg/ml) in DMEM containing 10% FBS. On day 2 and thereafter, DMEM containing 10% FBS and insulin (1 mg/ml) only was subsequently replaced every 2 days.
3T3-L1 preadipocytes or 8-day post-differentiation adipocytes were treated with 10 ng/ml TNF-α, eotaxin, IL-4, IL-5, or 100 pg/ml leptin for 30 min, 1, 2, 4, 8, 12, and 24 hrs, depending on the experimental procedures.
Four-week-old lean C57BL/6 mice were purchased from Hyochang Science (Daegu, Korea). The mice were fed a standard chow or high-fat (40% beef tallow) diet until the age of 26-weeks, then half of the high-fat group was converted to feed on the chow diet until 30 weeks. All mice were housed in cages on a 12-h day, 12-h night cycle, temperature, 22 ± 1℃; humidity, 55 ± 5%.
Mice were killed under ether anesthesia. Blood was collected in EDTA-coated tubes, and serum was separated by centrifugation (500 × g, 4℃, 10 min). Epididymal and visceral adipose tissue were collected by dissection, weighed, quickly frozen by immediate immersion in liquid nitrogen, and stored at -70℃ for RNA isolation. The lungs were washed by flushing with phosphate-buffered saline (PBS) through the tracheal cannula. The BAL fluid was centrifuged (500 × g for 10 min at 4℃), and the supernatant was stored at -20℃.
Total RNA was isolated from 3T3-L1 adipocytes using Trizol reagent. First-strand cDNA synthesis was performed with 1 mg of total RNA using Improm II reverse transcriptase (Promega).
Real-time RT-PCR analysis was performed using an Applied Biosystems 7500 Real Time PCR system (Applied Biosystems, Foster City, CA, USA). Samples (mixture of 2 × SYBR Green Mix, 0.2 µM of the appropriate primers, and cDNA) were incubated in the Applied Biosystems 7500 Real Time PCR system for an initial denaturation at 95℃ for 2 min, followed by 40 PCR cycles. Each cycle consisted of 95℃ for 15 s and 60℃ for 1 minute. Melting curve profiles (cooling the sample to 60℃ for 1 minute and heating slowly to 95℃ while continuously measuring fluorescence) were produced at the end of each PCR to confirm amplification of specific transcripts. The oligonucleotide primers for the experiments were: beta-actin forward; 5'-AGC CAT GTA CGT AGC CAT CC-3' and reverse; 5'-TCC CTC TCA GCT GTG GTG GTG AA-3', eotaxin forward; 5'-GAA TCA CCA ACA ACA GAT GCA C-3' and reverse; 5'-ATC CTG GAC CCA CTT CTT CTT-3'.
To investigate the changes in eotaxin expression during 3T3-L1 adipocyte differentiation, we collected cells every other day after inducing differentiation in 3T3-L1 preadipocytes and measured expression levels of eotaxin mRNA and protein secretion. Eotaxin gene expression levels in preadipocytes were dramatically reduced after 2 days of treatment with differentiation-inducing media, but eotaxin levels increased gradually starting from day 4, in which differentiation-inducing media was removed and differentiation proceeded with insulin only (Fig. 1A). Compared to differentiation at day 2, eotaxin levels were 4-fold higher at day 6 and 5.8-fold higher at day 8. Similarly, secreted eotaxin protein levels in media were reduced during the initial phase of differentiation, but the level was 4.9-fold higher at day 6 of differentiation (Fig. 1B).
To understand why eotaxin levels decreased dramatically following treatment with differentiation-inducing media in preadipocytes, we cultured preadipocytes under different media conditions (FBS: DMEM+ 10% FBS; insulin: Ins; isobutylmethylxantine: IBM-X; dexamethasone: dex) for 24 hours, then assessed their eotaxin mRNA levels and changes in secreted serum eotaxin levels. As a result, eotaxin mRNA and protein levels both increased with insulin stimulation, but their levels decreased 8-11-fold following IBMX and dex stimulation (Fig. 2).
TNF-α, a strong inducer of inflammation, induces the synthesis of eotaxin in several cell types [19]. We stimulated preadipocytes and fully differentiated adipocytes with 10 ng/ml TNF-α and analyzed eotaxin mRNA levels and protein accumulation in the media at several time points after induction to elucidate the differences in eotaxin expression following TNF-α treatment. As shown in Fig. 3A and B, eotaxin mRNA levels and protein secretion levels in differentiated adipocytes increased up to 40-fold following TNF-α stimulation. However, when preadipocytes were stimulated with TNF-α, eotaxin mRNA levels and protein secretion levels did not increase significantly (Fig. 3C, D).
When differentiated 3T3-L1 adipocytes were treated with 10 ng/ml of recombinant eotaxin, eotaxin mRNA levels were highest at 2 h after treatment. The amount of eotaxin mRNA expression from adipocytes was 2.97-fold and 4.9-fold higher than that of the control, respectively (Fig. 4).
To indirectly investigate the effect of eosinophils infiltrating adipose tissue on adipocytes, differentiated 3T3-L1 adipocytes were treated with 10 ng/ml IL-4 or IL-5 secreted from eosinophils, and eotaxin mRNA levels and the amount of secreted eotaxin protein in media were measured at different time points. Eotaxin mRNA levels increased gradually due to IL-4; its levels were 3.8-fold higher at 8 h compared to the control and continued increasing (Fig. 5A). After a 24-h treatment with IL-4, secreted eotaxin levels in media were 2.8-fold higher than that of the control (Fig. 5C). After IL-5 treatment, the eotaxin mRNA level was highest at 2 h and then decreased gradually. However, it increased again at 24 h (Fig. 5B). In contrast, secreted eotaxin levels in media after 24 h of IL-5 treatment were not significantly different than those of media without the treatment (Fig. 5D).
Exogenous leptin modulates the allergic airway response in mice [20], so we wondered about the effect of leptin on eotaxin expression in adipocytes. We treated differentiated adipocytes with 100 pg/ml of leptin and measured eotaxin mRNA levels and secreted eotaxin levels in media at different time periods. The results showed that the mRNA level at 4 h was 2.6-fold higher than the control and then decreased gradually. But, eotaxin increased again at 24 h to 1.6-fold higher than that of the control. The amount of secreted eotaxin during different time periods confirmed that levels increased gradually from 4 to 24 h (Fig. 6A). However, the secreted eotaxin level was not significantly higher than that of mRNA (Fig. 6B).
Four-week-old C57BL/6 mice were fed a standard normal diet for 1 week to adapt. Then, the mice labeled "HFD" were switched to a HFD, which included 40% beef tallow (Table 1) and were maintained on this diet for 30 weeks, so the weight gain was sufficiently large. "HFD→ND" labeled mice were fed a HFD for 26 weeks and then switched to a normal diet for 4 weeks to induce weight loss. The mice labeled "CON", were standard mice, which maintained a normal diet for the entire period (controls). The HFD group had about a 31.2% greater weight gain compared to the CON group, and the HFD→ND group had about a 17% weight loss compared to the HFD group (Table 2).
Comparing the change in eotaxin mRNA expression levels revealed that epididymal adipose tissue eotaxin levels and those of visceral adipose tissue were different. First, the HFD and HFD→ND groups both had reduced eotaxin mRNA levels in epididymal adipose tissue compared to the CON, and the HFD and HFD→ND groups were in a similar condition (Fig. 7A). In contrast, the HFD group had about 2.6-fold higher eotaxin mRNA levels in visceral adipose tissue than the CON group, and the HFD→ND group had about 5.4-fold lower compared to the HFD group (Fig. 7B).
Eotaxin protein levels in mice serum were different from eotaxin mRNA levels in adipose tissue. In adipose tissues, the HFD→ND group had the lowest eotaxin mRNA levels in both visceral adipose tissue and epididymal adipose tissue, but the HFD→ND group had about 1.3-fold higher levels of eotaxin protein in serum than the CON group (Fig. 8A).
Comparing both the HFD and HFD→ND groups with CON with regard to BALF eotaxin levels will bring us closer to understanding the relationship between obesity and asthma. The patterns of BALF eotaxin levels among the CON, HFD, and HFD→ND groups were similar to those of serum eotaxin (Fig. 8B).
We were able to confirm that eotaxin expression increased with the progression of 3T3-L1 differentiation in adipocytes. Furthermore, we observed an increase in eotaxin expression in 3T3-L1 adipocytes upon treatment with Th1/Th2 cytokines and chemokines such as TNF-α, eotaxin, IL-4, IL-5, and leptin. The level of eotaxin mRNA expression in visceral adipose tissues of HFD mice was much higher than the other groups, whereas the HFD→ND group mice showed decreasing levels of mRNA expression. All mice showed insignificant differences in eotaxin levels in epididymal adipose tissue, serum, and BALF.
The results of eotaxin expression studies on 3T3-L1 adipocytes to date have received several opinions. In past studies, eotaxin mRNA expression was higher in preadipocytes than in adipocytes and increased with LPS stimulation in both preadipocytes and adipocytes [17]. Therefore, we examined eotaxin mRNA expression and protein secretion levels at different stages of differentiation to determine eotaxin expression during 3T3-L1 adipocyte differentiation. Interestingly, our results showed that eotaxin expression levels decreased significantly at the early differentiation phase when IBMX and dex, which are differentiation induction components, were in the media. However, after removing the dex and IBMX, eotaxin levels increased gradually with differentiation in media with only insulin to induce differentiation. Dex is a synthetic glucocorticoid agonist that reduces inflammatory symptoms and immune reactions by stimulating the glucocorticoid receptor pathway. IBMX is a cAMP-phosphodiesterase inhibitor that reduces inflammatory symptoms and immune reactions by stimulating a cAMP-dependent protein kinase pathway, similar to dex [21]. Thus, reduced eotaxin levels in differentiation media were due to dex and IBMX. Consequently, this result explains the increased eotaxin levels after removing dex and IBMX. These data confirmed previous results stating that eotaxin mRNA levels are higher in preadipocytes than adipocytes [17], and suppressing eotaxin mRNA expression could be hypothesized as an effect of dex and IBMX anti-inflammatory activities during the induction of differentiation (Fig. 2).
TNF-α is a primary inflammatory cytokine and a strong inducer of inflammation. Treating adipocytes with TNF-α is an indirect method to determine their response to conditions similar to low chronic systemic inflammation present in obese subjects and TNF-α secretion from infiltrating macrophages. We determined that eotaxin mRNA and secreted protein levels in both preadipocytes and adipocytes increased following TNF-α stimulation, but the amount of eotaxin mRNA and protein secretion levels in preadipocytes were lower than that in adipocytes, indicating that the chances of inflammation inducing cells, which retain eotaxin receptors such as eosinophils, infiltrating adipose tissue increase with increased eotaxin expression.
Infiltrating cells, such as macrophages and eosinophils, occur in adipose tissue in addition to existing adipocytes. Obesity is thought to be responsible for the chemotaxis and infiltration of inflammation inducing cells, which mediate multiple metabolic diseases by inducing chronic low inflammation. Eotaxin is a chemokine expressed in both macrophages and eosinophils and, thus, treating adipocytes with eotaxin is an indirect method to determine the interaction between adipocytes in adipose tissue and stromal vascular cells. As expected, 3T3-L1 cells expressed more eotaxin mRNA following exogenous eotaxin stimulation. This indicated an increase in eotaxin mRNA expression in response to eotaxin stimulation, providing grounds for further studies with adipocyte and eosinophil co-cultures. This effect occurred much faster than with TNF-α treatment, and a continuous 2 hrs of exposure did not affect expression. This result indicates the possibility of a signaling pathway independent of that of TNF-α and presents a need for further studies on the expression mechanism of eotaxin in adipocytes.
A variety of cell types are responsible for eotaxin production, and eotaxin is produced following IL-4 stimulation in normal human lung fibroblast cells, indicating that Th2 cytokines may regulate eotaxin production in lung fibroblasts [22]. So, we wondered about eotaxin synthesis in adipocytes following stimulation by Th2 cytokines such as IL-4 and IL-5, which are secreted by eosinophils [23,24]. When adipocytes were treated with IL-4, eotaxin mRNA expression increased significantly, and eotaxin secretion was also much higher, indicating the possibility of an increased inflammatory response in adipose tissues due to a synergetic effect between Th2 cytokines and adipocytes. Otherwise, adipocytes treated with IL-5 had increased eotaxin mRNA levels but not protein secretion levels. These results lead us to examine the different mechanisms of eotaxin synthesis in adipocytes following stimulation by Th2 cytokines, which can be helpful for understanding the link between obesity and asthma with eotaxin as a mediator.
Some of the proinflammatory effects of adipose tissue have been attributed to the hormone leptin, which is produced by adipose tissue [1]. Studies of exogenous leptin modulating allergic airway responses in mice indicate the possibility of a relationship between obesity and asthma through leptin [21]. However, greater airway hyper-responsiveness and higher eotaxin levels were similarly observed after obese mice with a genetic deficiency in leptin were exposed to ozone [25]. These results show that leptin alone is insufficient to explain the relationship between obesity and asthma and led to a focus on the role of eotaxin. Therefore, we treated differentiated adipocytes with leptin. As a result, both eotaxin mRNA levels and the amount of secreted protein in adipocytes increased over time. Leptin, which increases with obesity, is a hormone that mediates obesity and inflammation. The result of increased eotaxin level in adipocytes due to leptin stimulation showed that the interaction between leptin and eotaxin has an effect on intensifying airway inflammation. This kind of interaction may play a significant role in the relationship between obesity and asthma. Therefore, more in-depth research on this mechanism is necessary.
Prior studies have shown increased eotaxin mRNA expression in adipose tissues of diet-induced obese mice when compared with that of normal mice. However, this study used obese mice that subsequently underwent weight loss through a normal diet. It has been reported that the visceral fat of obese human subjects shows significantly increased eotaxin mRNA expression compared with that of subcutaneous fat. Furthermore, unlike other CC chemokines, eotaxin has not shown any differences in expression in the subcutaneous fat of obese and normal human subjects [26].
We investigated eotaxin expression in visceral and epididymal adipose tissue and showed significant differences in expression in obese, weight-loss, and normal mice with regards to visceral fat, whereas little difference in expression was observed in epididymal fat. This result shows that adipose tissue may have differing functions depending on location. Further studies including determining CD68 and macrophage markers to assess infiltrating macrophages in tissue and determining eotaxin mRNA expression in each type of stromal vascular cells would allow for a deeper approach to understand the effects of infiltrating cells in adipose tissues.
Other studies have shown increased levels of eotaxin in serum and BALF of obese mice than normal mice; however, our results showed minimal differences in eotaxin levels in the three mice groups studied. Serum and BALF eotaxin levels are related to the onset and severity of asthma; thus, further research is required to attain accurate data.
In conclusion, we have shown that eotaxin expression in 3T3-L1 adipocytes increased during adipocyte differentiation and that an increase in eotaxin expression was observed following inflammatory stimulation with TNF-α, IL-4, IL-5, and leptin. Moreover, experiments on diet-induced obese mice show increased eotaxin mRNA expression in visceral adipose tissue due to a HFD and decreased levels upon weight loss. These results indicate a close relationship between eotaxin expression and obesity as well as weight loss and thus indirectly shows a relation to asthma.
Figures and Tables
Fig. 1
Changes in eotaxin expression during 3T3-L1 adipocyte differentiation. (a) 3T3-L1 adipocytes were collected every other day during differentiation, and the relative amount of eotaxin mRNA was detected using real-time RT-PCR. (b) 3T3-L1 adipocyte supernatants were collected every other day during differentiation, and the eotaxin secretion level was detected with an ELISA kit (D0: preadipocytes; D2-D10: 2-10 days after inducing differentiation; *P < 0.05, **P < 0.01, ***P < 0.001).

Fig. 2
Change in eotaxin expression in 3T3-L1 preadipocytes due to differentiation components. Preadipocytes were treated with several different differentiation media for 24 hours. (a) The relative amount of eotaxin mRNA was determined by real-time RT-PCR, and (b) eotaxin secretion was detected with an ELISA kit (fetal bovine serum [FBS]: DMEM + 10% FBS, FBS + insulin [Ins]: DMEM + 10% FBS + Ins 1 µg/ml, FBS + IBMX: DMEM + 10% FBS + IBMX 0.5mM, FBS + dexamethasone [DEX]: DMEM + 10% FBS + Dex 0.25 mM, DM: DMEM + 10% FBS + Ins 1µg/ml + IBMX 0.5 mM + Dex 0.25 mM; *P < 0.05, **P < 0.01, ***P < 0.001).

Fig. 3
Changes in eotaxin expression in 3T3-L1 preadipocytes and adipocytes after stimulation with TNF-α. (a) At day 8 after inducing differentiation, a time course treatment with 10 ng/ml TNF-α was conducted for 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours, the relative amount of eotaxin mRNA was detected by real-time RT-PCR, and (b) eotaxin secretion were detected with an ELISA kit. (c) Time-course treatment with 10 ng/ml TNF-α on preadipocytes was conducted for 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours, and (d) eotaxin secretion level was detected with an ELISA kit (*P < 0.05, **P < 0.01, ***P < 0.001).

Fig. 4
Changes in eotaxin expression in differentiated 3T3-L1 adipocytes following treatment with recombinant eotaxin. At 8 days after inducing differentiation, adipocytes were treated with 10 ng/ml recombinant eotaxin, and the cells were collected in a time course. The relative amount of eotaxin mRNA expression is shown (**P < 0.01).

Fig. 5
Changes in eotaxin expression in 3T3-L1 adipocytes following interleukin (IL)-4 and IL-5 treatment. At day 8 after inducing differentiation, a time course treatment of 10 ng/ml (a) IL-4 and (b) IL-5 was conducted for 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours, and the relative amount of eotaxin mRNA was detected by real-time RT-PCR. (c) At day 8 after inducing differentiation, 10 ng/ml IL-4 and (d) IL-5 were added for 24 hours, and eotaxin secretion levels were detected with an ELISA kit (*P < 0.05, **P < 0.01, ***P < 0.001).

Fig. 6
Changes in eotaxin expression in 3T3-L1 adipocytes following leptin treatment. (a) At day 8 after inducing differentiation, a time course treatment of 100 pg/ml leptin was conducted for 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours, and the relative amount of eotaxin mRNA was detected by real-time RT-PCR. (b) At day 8 after inducing differentiation, 100 pg/ml leptin was introduced for 4 hours, 8 hours, 12 hours, and 24 hours, and eotaxin secretion levels were detected with an ELISA kit (*P < 0.05, **P < 0.01, ***P < 0.001).
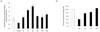
Fig. 7
Changes in adipose tissue eotaxin expression in different locations following diet-induced obesity and weight loss. The relative amount of eotaxin mRNA expression in (a) epididymal adipose tissues, and (b) visceral adipose tissues in the control (CON), HFD, and HFD→ND were observed by real-time RT-PCR (ND: normal diet; HFD: high-fat diet; HFD→ND: diet induced obesity and then induced weight loss by normal diet; *P < 0.05, **P < 0.01, ***P < 0.001).

References
1. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005. 115:911–919.

2. Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro, and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993. 197:1167–1172.
3. Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996. 271:7725–7730.

4. Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008. 104:1727–1735.

5. Li D, Wang D, Griffiths-Johnson DA, Wells TN, Williams TJ, Jose PJ, Jeffery PK. Eotaxin protein and gene expression in guinea-pig lungs: constitutive expression and upregulation after allergen challenge. Eur Respir J. 1997. 10:1946–1954.

6. Bartels J, Schlüter C, Richter E, Noso N, Kulke R, Christophers E, Schröder JM. Human dermal fibroblasts express eotaxin: molecular cloning, mRNA epression, andidentification of eotaxin sequence variants. Biochem Biophys Res Commun. 1996. 225:1045–1051.

7. Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006. 91:256–261.

8. Hashimoto I, Wada J, Hida A, Baba M, Miyatake N, Eguchi J, Shikata K, Makino H. Elevated serum monocyte chemoattractant protein-4 and chronic inflammation in overweight subjects. Obesity (Silver Spring). 2006. 14:799–811.

9. Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001. 164:2045–2050.

10. Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002. 122:1256–1263.

11. Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999. 54:396–402.

12. Huang SL, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy. 1999. 29:323–329.

14. Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomized controlled study. BMJ. 2000. 320:827–832.

15. Choi KM, Kim JH, Cho GJ, Baik SH, Park HS, Kim SM. Effect of exercise training on plasma visfatin and eotaxin levels. Eur J Endocrinol. 2007. 157:437–442.

16. Do MS, Jeong HS, Choi BH, Hunter L, Langley S, Pazmany L, Trayhurn P. Inflammatory gene expression patterns revealed by DNA microarray analysis in TNF-alpha-treated SGBS human adipocytes. Yonsei Med J. 2006. 47:729–736.

17. Poulain-Godefroy O, Froguel P. Preadipocyte response and impairment of differentiation in an inflammatory environment. Biochem Biophys Res Commun. 2007. 356:662–667.

18. Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007. 176:650–658.

19. Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010. 159:617–625.

20. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005. 115:103–109.

21. Ntambi JM, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000. 130:3122S–3126S.

22. Suzuki T, Arakawa H, Mizuno T, Muramatsu K, Tadaki H, Takizawa T, Mochizuki H, Tokuyama K, Matsukura S, Morikawa A. Differential regulation of eotaxin expression by dexamethasone in normal human Lung fibroblasts. Am J Respir Cell Mol Biol. 2008. 38:707–714.

23. Bjerke T, Gaustadnes M, Nielsen S, Schiøtz PO, Rudiger N, Reimert CM, Dahl R, Christensen I, Poulsen LK. Human blood eosinophils produce and secrete interleukin 4. Respir Med. 1996. 90:271–277.

24. Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994. 179:703–708.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download