Abstract
The prevalence, virulence potential, and antibiotic resistance of ophthalmic Staphylococcus pseudintermedius (SP) isolated from dogs were examined. Sixty-seven Staphylococcus species were isolated from ophthalmic samples and surveyed for species-specific sequences in the Staphylococcus intermedius group (SIG) nuclease gene (SInuc), exfoliative toxin gene for SIG (siet), and antibiotic resistance genes (blaZ and mecA). PCR-restriction fragment length polymorphism analysis of the pta gene was also performed. Fifty isolates were identified as SIG strains, all of which were found to be SP. The blaZ gene was detected in 42 of the 50 SP strains and mecA gene was observed in 18 of the 50 SP strains. The 50 SP strains were most susceptible to amoxicillin/clavulanic acid (94%) and chlorampenicol (70%), and highly resistant to tetracycline (94%) and penicillin (92%). It was also found that 16 (88.9%) mecA-positive SP strains were resistant to oxacillin, tetracycline and penicillin. All mecA-positive SP were resistant to more than four of the eight tested antibiotics and therefore considered SP with multi-drug resistance (MDR). Our results indicate a high prevalence of antibiotic resistance genes in ophthalmic SP along with a close relationship between MDR SP strains and the mecA gene. Based on our findings, judicious administration of antibiotics to companion dogs is necessary.
The Staphylococcus intermedius group (SIG), previously called 'Staphylococcus (S.) intermedius', is an opportunistic bacterium that is most frequently associated with skin diseases such as pyoderma and otitis externa in dogs [9]. The SIG inhabiting skin includes three species: S. pseudintermedius (SP), S. intermedius, and S. delphini. These coagulase-positive staphylococci (CoPS) have similar phenotypes and differentiation among CoPS has relied on molecular identification techniques [17]. In companion dogs, the SIG seems to consist solely of SP [17,31]. The SIG is also known to be a primary pathogen that causes canine ocular diseases such as keratitis and abscess; however, little information is available about the prevalence of SP in canine ophthalmic microflora [24,27,28].
The SIG bacterium disrupts cell-to-cell adhesion of eukaryotic cells during skin infection by producing a 27-kDa exfoliative toxin (siet) that targets a desmosomal cell-cell adhesion molecule in the superficial epidermis (desmoglein 1) and facilitates percutaneous bacterial invasion of mammalian skin [15,23]. In recent studies, methicillin resistance and multi-drug resistance (MDR) in animal pathogenic Staphylococcal spp. have been implicated in potential zoonotic infections among humans [20,21,32]. In particular, mecA has been closely linked with MDRSP or methicillin-resistant SP (MRSP), thereby decreasing the arsenal of effective antibiotics [6,10,30,31]. The zoonotic transfer of MDRSP and MRSP could critically impact public health. The indiscriminate use of broad-spectrum systemic or topical antibiotics for therapeutic purposes and as a prophylaxis against ocular disease could increase bacteria resistance and zoonotic potential [27]. However, limited data on the prevalence of ophthalmic MDRSP and MRSP is available in veterinary practice and literature. The purpose of this study was to evaluate the prevalence and antibiotic resistance of SP isolates from canine eyes, and identify potential virulence genes using molecular techniques.
Sixty-seven staphylococcal strains were isolated from individual canine eyes between March 2005 and September 2007 using a standard bacteriological method. Collection of the isolates was performed at the Veterinary Medical Teaching Hospital of Konkuk University (Korea). Thirty-three dogs referred for ocular disease were treated or suspected of having been treated with antibiotics. Ocular diseases included corneal ulcer, conjunctivitis, keratoconjunctivitis sicca, uveititis, keratoconjunctivitis, blepharitis, glaucoma, corneal degeneration, conjunctival hyperemia, ocular hemorrhage, cataract, progressive retinal atrophy, and retinal detachment. Other dogs were referred for non-ocular disease, and no information was available. Two cotton-tipped culture swabs were taken from each dog. Preliminarily screening was conducted using standard microbiological procedures including a phenotypical test, Gram staining, and a catalase test to differentiate staphylococcal species.
Bacterial DNA extraction was performed by inoculating Luria-Bertani (LB) medium with a single colony and incubating overnight at 37℃. Next, 1.5 mL of the cultured medium was centrifuged at 12,000 × g for 1 min. The bacterial pellet was then suspended in 500 µL of Tris-EDTA (10 mM Tris, pH 8.0, and 0.1 mM EDTA) buffer and DNA was extracted using a commercial kit (Optima Scientific, Japan).
PCR-based confirmation of SIG was carried out with species-specific primers targeting the SIG nuclease (nuc) gene as previously described [2]. Additionally, PCR reactions specific for the siet, mecA, and blaZ genes were performed to identify exfoliative toxin production, oxacillin resistance, and penicillin resistance, respectively, as previously described [14,22]. Five primer pairs were used for our multiplex PCR and the blaZ gene-targeting primers were modified to distinguish the amplicon of interest from other amplicons based on size. The oligonucleotide primer sequences and PCR programs are summarized in Table 1.
After primer modification, the amplicon size of the blaZ gene was about 700 bp. The PCR-amplified products were subsequently analyzed by direct DNA sequencing (Macrogen, Korea). All of the multiplex PCR amplicons were the expected size when the DNA templates were 10~10-1 ng. The amplicons were between 125 and 700 bp in size, and correctly differentiated by agarose gel electrophoresis.
For this study, we mixed 2 ng of the template DNA from the 67 samples with 12.5 µL of 2× EF-Taq PCR Pre-Mix (SolGent, Korea). Next, 2.5 pmol of 16S primers or 10 pmol of the other primers were added to amplify the gene-specific PCR fragment. The PCR conditions are described in Table 1. To identify the PCR product, we separated 10 µL of the reaction product in a 2% agarose gel by electrophoresis and stained with ethidium bromide for visualization. A 16S rDNA primer pair specific for Staphylococcus functioned as the internal control, confirming the amplification efficiency (Fig. 1).
PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the pta gene was performed for SP identification. Following a previously described protocol [1], we used pta primers and MboI restriction enzyme. The concentrations of the primers, template DNA, and restriction enzyme were modified along with the PCR product volume to increase the reaction efficiency. We mixed 2 ng of template DNA with 15 µL of 2× SolGent EF-Taq PCR Pre-Mix, 10 pmol of the pta primers, and 11 µL of distilled water. The PCR conditions are described in Table 1. After post-extension, we mixed 5 µL of the PCR product with 2 µL of 10× NE buffer 4 (50 mM potassium-acetate, 20 mM Tris-acetate, 10 mM Mg-acetate, 1 mM DTT, pH 7.9), 1 U of MboI restriction enzyme, and 12 µL of distilled water. The solution was then incubated at 37℃ for 4 h. SP was identified based on the detection of 107 and 203 bp pta gene fragments (Fig. 2).
An antibiogram analysis was carried out following the guidelines of the Clinical and Laboratory Standards Institute [5]. Eight commonly prescribed antibiotics were tested [amoxicillin/clavulanic acid (30 µg), oxacillin (1 µg), chloramphenicol (30 µg), gentamycin (10 µg), erythromycin (15 µg), sulfametoxazole/trimethoprim (25 µg), tetracycline (30 µg), and penicillin (10 µg)] using commercially available BD BBL Sensi-Disc disks (BD Diagnostics, USA).
According to the multiplex PCR results, eight groups were identified based on the prevalence of the SInuc, siet, blaZ, and mecA genes (Fig. 1; Table 2). The 16S rDNA gene amplicon internal control was clearly positive for all 67 strains. Among these, 50 strains (74.6%) in groups 1 to 3 contained the SInuc gene specific for SIG. Out of the 50 ophthalmic SP strains, 25 were isolated from dogs with ocular disease and the other 25 were isolated from dogs without ocular disease. Similar to a previous report [26], all SIG strains were confirmed as SP according to the PCR-RFLP assay and all SP-encoded exfoliative toxin-producing genes were predominantly found in the ophthalmic SP strains. None of the SInuc gene-negative strains were identified as SP. The blaZ antibiotic resistance gene was present in 46 of the 50 SP strains (94%). Among these, 18 strains (36%) also contained the mecA gene. Notably, none of the blaZ gene-negative strains expressed the mecA gene (Table 2). Prevalence of the mecA gene was twice as high in SP isolated from dogs with ocular disease (12/25, 48%) compared to ones without (6/25, 24%).
Susceptibility to eight commonly used antibiotics of the 50 SP isolates is presented in Table 3. More than 50% of the SP isolates were resistant to tetracycline, penicillin, sulfamethoxazole/trimethoprim, erythromycin, and gentamycin. The levels of susceptibility were highest for amoxicillin/clavulanic acid and chloramphenicol. Seventeen of the 50 (34%) SP strains were resistant to oxacillin, indicating a high prevalence of MRSP (Table 3).
There was significant correlation between genes conferring antibiotic resistance (mecA and blaZ) and antibiotic susceptibility (penicillin and oxacillin). The 18 mecA-ositive and blaZ-positive strains were 100% resistant to penicillin. Most of the 18 mecA-positive strains were resistant to oxacillin (88.9%). However, only 37% of the 46 blaZ-positive strains were resistant to this reagent. Based on these results, antimicrobial resistance of the 50 ophthalmic SP isolates was re-evaluated based on the presence of the mecA gene. Among the mecA-positive isolates (18/50), most oxacillin-resistant SP strains (16/18) were also resistant to multiple antibiotics such as tetracycline, sulfamethoxazole/trimethoprim, erythromycin, and gentamycin. Additionally, two of the mecA-positive SP strains were oxacillin susceptible and one mecA-negative SP strain was resistant to oxacillin (Table 4). All mecA-positive SP isolates were resistant to more than four of the eight tested antibiotics, indicating an association of MDRSP with mecA gene-ositive SP (Fig. 3).
All ophthalmic SIG isolates (50/67, 74.6%) in this study were re-identified as SP. These results were similar with ones from a study on skin-derived SIG [30]. It has been suggested that SP is highly host-specific and foot-to-eye transmission is possible. Universal prevalence of exfoliative toxin-producing genes among the SP strains examined in the current investigation strongly implies the high possibility that these bacteria can cause ophthalmic disease [14].
The prevalence of MRSP has been commonly studied in dogs and cats with skin disease [26]. According to a recent report, the prevalence of MRSP in North America and Canada is 0%~7% [26]. These rates are slightly elevated in other countries with 12.7% in China [29], 11.4% in Japan [16], and 17.6% in Korea [31]. In the present investigation, the MRSP rate was 34%, which was higher than that previously reported in studies of skin. The frequent use of broad-spectrum antibiotics as canine ocular medications could have contributed to the relatively high prevalence of MRSP we observed although the exact history of antibiotic administration was not obtained for our survey.
In the current study, 46 out of 50 (92%) SP isolates possessed the blaZ gene while 18 out of 50 (36%) had the mecA gene. These two genes are specific for Staphylococcus penicillin- and oxacillin-like β-lactam antibiotic resistance. Furthermore, prevalence of the mecA gene was approximately two-times higher among dogs with ocular diseases. These results indicate a high rate of antibiotic resistance genes in ophthalmic SP. Among the 50 SP strains, 16 (32%) of the oxacillin-resistant isolates possessing the mecA gene were twice as likely to be resistant to the three most susceptible antibiotics, suggesting that the mecA gene is closely associated with MDR [11,12]. The increased prevalence of SP harboring the mecA gene could thus impact patient health since the antibiotic choices for therapy would be narrowed.
Two out of the 18 SP isolates harboring the mecA gene were susceptible to methicillin and therefore classified as pre-MRSP [3]. Pre-MRSP strains contain a functionally intact mecR1-mecI regulatory region. As a result, mecA expression might be strongly repressed. Under antibiotic pressure, such pre-MRSPs tend to constitutively produce penicillin-binding protein (PBP2a) and acquire increased resistance to methicillin.
One strain was negative for the mecA gene but methicillin-resistant (1/32, 3.1%). It is possible that the hyperproduction of β-lactamase or PBPs alternatively produced by another type of antibiotic resistance gene could help the bacteria resist oxacillin-like β-lactam antibiotics in the absence of the mecA gene [8,25]. In 2011, a novel mecA homologue designated mecC (or mecALGA251) was described [7]. Because of its highly divergent sequence, this gene cannot be detected by routine molecular assays designed to identify mecA [13,18]. This suggests that a different mec element not detectable by conventional mecA-specific PCR could be involved. Further study of these mechanisms will be helpful for developing new drugs that target β-lactam-resistant Staphylococcus.
In this study, we described a multiplex PCR procedure to detect clinically relevant antibiotic resistance genes in ophthalmic SP isolates. Although classical susceptibility testing methods are relatively simple, the results may be highly dependent on experimental conditions. Furthermore, there is no certain way to determine antibiotic concentrations in ocular tissues during topical therapy. Results of our multiplex PCR assay were comparable to ones from the standard susceptibility test performed in this study. A recent Clinical and Laboratory Standards Institute (CLSI) recommendation [5] is germane to the discrepancy observed between the mecA gene prevalence and oxacillin resistance we observed. According to this recommendation, strains of S. aureus and S. lugdunensis should be reported as oxacillin-resistant if either disc result indicates resistance when both cefoxitin and oxacillin are tested. Indeed, some studies have reported that a 30-µg cefoxitin disc is more sensitive than a 1-µg oxacillin disc when detecting mecA-mediated oxacillin resistance among staphylococci [4,19]. However, pre- MRSP and mecA-negative oxacillin-resistant SP strains were also sensitive to cefoxitin in the present study (data not shown). Thus, we recommend that both an oxacillin disc test and PCR detection should be performed together to identify ophthalmic MRSP. In addition, multiplex PCR detection could provide more information for developing an effective antibiotic therapy.
In conclusion, results of our study demonstrated that ophthalmic MDRSP and MRSP were prevalent in dogs. The phenotypic and genotypic comparison of antibiotic resistance showed that 88.9% of all the mecA-positive SP isolates was resistant to oxacillin. Notably, all the mecA-positive and oxacillin-resistant SP isolates displayed MDR. Based on our findings, we strongly recommend that judicious selection of antibiotics in companion dogs is necessary to prevent MDR.
Figures and Tables
Fig. 1
Results of the multiplex PCR assay. Detection of the SInuc (125 bp), siet (359 bp), mecA (532 bp), and blaZ (700 bp) genes. A highly conserved region of 16S rDNA (252 bp) was used as an internal positive control. Marker, 100 bp ladder; SIG, S. intermedius group.
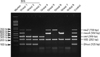
Fig. 2
PCR-restriction fragment length polymorphism (RFLP) analysis of the SP pta gene. M, 100 bp ladder; 1, S. pseudintermedius (SP; 213 and 107 bp); 2, S. aureus (156 and 164 bp); 3, S. intermedius or S. delphini (Others, 320 bp); 4, Not S. intermedius or S. aureus.

Fig. 3
Correlation of methicillin resistance and multi-drug resistance in the 50 ophthalmic SP isolates.

Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2013R1A1A2006152).
References
1. Bannoehr J, Franco A, Iurescia M, Battisti A, Fitzgerald JR. Molecular diagnostic identification of Staphylococcus pseudintermedius. J Clin Microbiol. 2009; 47:469–471.

2. Baron F, Cochet MF, Pellerin JL, Ben Zakour N, Lebon A, Navarro A, Proudy I, Le Loir Y, Gautier M. Development of a PCR test to differentiate between Staphylococcus aureus and Staphylococcus intermedius. J Food Prot. 2004; 67:2302–2305.

3. Berger-Bächi B, Rohrer S. Factors influencing methicillin resistance in staphylococci. Arch Microbiol. 2002; 178:165–171.

4. Broekema NM, Van TT, Monson TA, Marshall SA, Warshauer DM. Comparison of cefoxitin and oxacillin disk diffusion methods for detection of mecA-mediated resistance in Staphylococcus aureus in a large-scale study. J Clin Microbiol. 2009; 47:217–219.

5. CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. CLSI document M100-S20. Wayne: Clinical and Laboratory Standards Institute;2010.
6. Descloux S, Rossano A, Perreten V. Characterization of new staphylococcal cassette chromosome mec (SCCmec) and topoisomerase genes in fluoroquinolone- and methicillin-resistant Staphylococcus pseudintermedius. J Clin Microbiol. 2008; 46:1818–1823.

7. García-Álvarez L, Holden MTM, Lindsay H, Webb CR, Brown DFJ, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RLR, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. Methicillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011; 11:595–603.

8. Griffeth GC, Morris DO, Abraham JL, Shofer FS, Rankin SC. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet Dermatol. 2008; 19:142–149.

10. Jones RD, Kania SA, Rohrbach BW, Frank LA, Bemis DA. Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1,772 samples (2001-2005). J Am Vet Med Assoc. 2007; 230:221–227.

11. Khan AU, Sultan A, Tyagi A, Zahoor S, Akram M, Kaur S, Shahid M, Vaishnavi CV. Amplification of mecA gene in multi-drug resistant Staphylococcus aureus strains from hospital personnel. J Infect Dev Ctries. 2007; 1:289–295.

12. Kizerwetter-Swida M, Chrobak D, Rzewuska M, Binek M. Antibiotic resistance patterns and occurrence of mecA gene in Staphylococcus intermedius strains of canine origin. Pol J Vet Sci. 2009; 12:9–13.
13. Laurent F, Chardon H, Haenni M, Bes M, Reverdy ME, Madec JY, Lagier E, Vandenesch F, Tristan A. MRSA harboring mecA variant gene mecC, France. Emerg Infect Dis. 2012; 18:1465–1467.
14. Lautz S, Kanbar T, Alber J, Lämmler C, Weiss R, Prenger-Berninghoff E, Zschöck M. Dissemination of the gene encoding exfoliative toxin of Staphylococcus intermedius among strains isolated from dogs during routine microbiological diagnostics. J Vet Med B Infect Dis Vet Public Health. 2006; 53:434–438.

15. Nishifuji K, Sugai M, Amagai M. Staphylococcal exfoliative toxins: "molecular scissors" of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci. 2008; 49:21–31.

16. Onuma K, Tanabe T, Sato H. Antimicrobial resistance of Staphylococcus pseudintermedius isolates from healthy dogs and dogs affected with pyoderma in Japan. Vet Dermatol. 2012; 23:17–22.
17. Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Reclassification of phenotypically identified Staphylococcus intermedius strains. J Clin Microbiol. 2007; 45:2770–2778.

18. Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R, Coleman DC. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011; 55:3765–3773.

19. Skov R, Smyth R, Larsen AR, Bolmstrôm A, Karlsson A, Mills K, Frimodt-Moller N, Kahlmeter G. Phenotypic detection of methicillin resistance in Staphylococcus aureus by disk diffusion testing and Etest on Mueller-Hinton agar. J Clin Microbiol. 2006; 44:4395–4399.

20. Soedarmanto I, Kanbar T, Ülbegi-Mohyla H, Hijazin M, Alber J, Lämmler C, Akineden Ö, Weiss R, Moritz A, Zschöck M. Genetic relatedness of methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolated from a dog and the dog owner. Res Vet Sci. 2011; 91:e25–e27.
21. Stegmann R, Burnens A, Maranta CA, Perreten V. Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J Antimicrob Chemother. 2010; 65:2047–2048.

22. Strommenger B, Kettlitz C, Werner G, Witte W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J Clin Microbiol. 2003; 41:4089–4094.

23. Terauchi R, Sato H, Hasegawa T, Yamaguchi T, Aizawa C, Maehara N. Isolation of exfoliative toxin from Staphylococcus intermedius and its local toxicity in dogs. Vet Microbiol. 2003; 94:19–29.

24. Tolar EL, Hendrix DV, Rohrbach BW, Plummer CE, Brooks DE, Gelatt KN. Evaluation of clinical characteristics and bacterial isolates in dogs with bacterial keratitis: 97 cases (1993-2003). J Am Vet Med Assoc. 2006; 228:80–85.

25. Tomasz A, Drugeon HB, de Lencastre HM, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989; 33:1869–1874.

26. van Duijkeren E, Catry B, Greko C, Moreno MA, Pomba MC, Pyörälä S, Ruzauskas M, Sanders P, Threlfall EJ, Torren-Edo J, Törneke K. Scientific Advisory Group on Antimicrobials (SAGAM). Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2011; 66:2705–2714.
27. Varges R, Penna B, Martins G, Martins R, Lilenbaum W. Antimicrobial susceptibility of Staphylococci isolated from naturally occurring canine external ocular diseases. Vet Ophthalmol. 2009; 12:216–220.

28. Wang L, Pan Q, Zhang L, Xue Q, Cui J, Qi C. Investigation of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Beijing, China. Vet Ophthalmol. 2008; 11:145–149.

29. Wang Y, Yang J, Logue CM, Liu K, Cao X, Zhang W, Shen J, Wu C. Methicillin-resistant Staphylococcus pseudintermedius isolated from canine pyoderma in North China. J Appl Microbiol. 2012; 112:623–630.

30. Yoo JH, Yoon JW, Lee SY, Park HM. High prevalence of fluoroquinolone- and methicillin-resistant Staphylococcus pseudintermedius isolates from canine pyoderma and otitis externa in veterinary teaching hospital. J Microbiol Biotechnol. 2010; 20:798–802.
31. Yoon JW, Lee KJ, Lee SY, Chae MJ, Park JK, Yoo JH, Park HM. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J Microbiol Biotechnol. 2010; 20:1764–1768.
32. Zubeir IE, Kanbar T, Alber J, Lämmler C, Akineden O, Weiss R, Zschöck M. Phenotypic and genotypic characteristics of methicillin/oxacillin-resistant Staphylococcus intermedius isolated from clinical specimens during routine veterinary microbiological examinations. Vet Microbiol. 2007; 121:170–176.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download