Abstract
Climate change induced by recent global warming may have a significant impact on vector-borne and zoonotic diseases. For example, the distribution of Japanese encephalitis virus (JEV) has expanded into new regions. We surveyed the levels of hemagglutination-inhibition (HI) antibodies against JEV (Family Flaviviridae, genus Flavivirus) in wild birds captured in Korea. Blood samples were collected from 1,316 wild birds including the following migratory birds: Oceanodroma castro (n = 4), Anas formosa (n = 7), Anas penelope (n = 20), Fulica atra (n = 30), Anas acuta (n = 89), Anas crecca (n = 154), Anas platyrhynchos (n = 214), Aix galericulata (n = 310), and Anas poecilorhyncha (n = 488). All were captured in 16 locations in several Korea provinces between April 2007 and December 2009. Out of the 1,316 serum samples tested, 1,141 (86.7%) were positive for JEV. Wild birds captured in 2009 had a higher seroprevalence of ant-JEV antibodies than those captured in 2007. Wild birds with an HI antibody titer of 1 : 1,280 or higher accounted for 21.2% (280/1,316) of the animals tested. These findings indicated that wild birds from the region examined in our study have been exposed to JEV and may pose a high risk for introducing a new JEV genotype into Korea.
Japanese encephalitis virus (JEV) is a mosquito-borne microorganism that causes encephalitis in humans and reproductive failure in pregnant sows [1,9]. JEV has been recently recognized as a reemerging pathogen as it has expanded into new territories such as the Torres Strait and Australia [5,10,18-20]. Based on a sequence analysis of the C/prM and E genes, JEV has been classified into five genotypes; their relationships have been characterized [1,7,17]. JEV genotype 1, which was distributed in limited regions such as Thailand and Cambodia before the 1990s, has expanded into northeastern Asian countries including Vietnam, China, Japan, and Korea [7,11,14,19,22]. Although the exact mechanisms for the expansion of this genotype are unknown, several factors such as bird migration, wind-blown mosquitoes, and the transport of animals infected with JEV have been suggested [11,13,21]. Additionally, climate changes caused by recent global warming and extreme weather patterns may have significantly impacted the transmission of vector-borne diseases such as JEV, West Nile virus (WNV), and tick-borne encephalitis virus (TBEV). This is because rapid weather and climate changes can directly or indirectly affect migratory birds and mosquitoes [12].
Sero-epidemiological studies are critical for predicting potentially important viral disease outbreaks and preventing the introduction of new JEV genotypes into Korea. Recently, Saito et al. [12] suggested that wild ducks captured in Hokkaido, Japan can transmit vector-borne viruses into new territories. Migratory birds may serve as viral reservoirs or amplifying hosts, but they do not develop clinical symptoms. Although migrating wild birds may be a major JEV vector, no epidemiological survey of JEV, which could provide valuable information for establishing control measures to prevent JEV outbreaks in swine and humans, has been properly conducted for wild birds in Korea. Thus, we performed a serological survey to determine the prevalence of antibodies against JEV in wild birds captured on the Korean peninsula.
A total of 1,316 blood samples were collected from wild birds in 16 locations of six provinces (Gyeonggi-do; 3 site, Gyeongsangnam-do; 1 site, Jeollanam-do; 4 sites, Jeollabuk-do; 3 sites, Chungcheongnam-do; 3 sites, Chungcheongbuk-do; 2 sites) of Korea between April 2007 and December 2009 for our seroprevalence study. All wild birds were lured by rice seed and captured using Cannon or Mist net. Blood sample from wing vein of each bird using sterile syringe of 3 mL was taken. And then the wild birds released after blood sampling. The nine species of wild birds tested in this study were Oceanodroma castro (four birds), Anas Formosa (seven), Anas Penelope (20), Fulica atra (30), Anas acuta (89), Anas crecca (154), Anas platyrhynchos (214), Aix galericulata (310), and Anas poecilorhyncha (488). Most of the wild birds captured were adults. Clotted blood samples were separated by 3,000× g, and the sera were stored at -20℃ until use.
Before performing the HI test, the sera were inactivated by incubating at 56℃ for 30 min. The KV1899 (genotype 1 strain) strain of JEV was used as the positive antigen for the HI test. This strain was isolated from Korean pig blood in 1999 and has been passed nine times in Vero cells after isolation. To estimate the JEV antibody prevalence in the wild bird sera, an HI test was performed in 96-well microplates (Corning, USA) using slightly modified standard methods [2,8]. A sucrose-acetone extraction method was used to prepare viral antigens from the brains of suckling mice infected with the Korean isolate of JEV strain KV1899. Briefly, 10 µL of the serum samples collected from wild birds and 50 µL of 4% bovine albumin were mixed with 40 µL of 25% kaolin (Sigma, USA) and incubated for 30 min to remove non-specific inhibitors at room temperature. After mixing, the kaolin was removed by centrifugation at 3,000× g for 15 min. The clear supernatant was then mixed with 5 µL of packed goose erythrocytes to remove any natural agglutinins. After incubating at 37℃ for 1 h, the treated sera were separated from the goose erythrocytes by 3,000× g at 4℃. The sera (25 µL) were diluted two-fold from 1 : 20 to 1 : 10,240 in round-bottom 96-well microplates (Corning, USA) and incubated with 8 hemagglutinating antigen (HA) units of JEV. After incubating at 37℃ for 1 h, 50 µL of 0.33% goose erythrocytes were added and the microplates were incubated for 30 min at 37℃. To confirm the test reliability, porcine control sera positive and negative for JEV were used in all HI tests. HI titer was expressed as the reciprocal of the highest dilution of serum showing complete hemagglutination inhibition. An HI titer of 1 : 20 or higher was considered positive.
JEV prevalence was estimated as the number of positive samples compared to the number of samples tested and expressed as a percentage. A 95% confidence interval (CI) was calculated for the estimated prevalence. Additionally, each titer was classified according to bird species, time (year), and place (capture location). The titers were estimated and compared using a chi-square test.
JEV seroprevalence as determined by our study is shown in Tables 1, 2, 3, and Figs. 1 and 2. The overall prevalence of antibodies against JEV was 86.7% (95% CI, 84.7~88.5%) for the 1,316 serum samples consisting of nine wild bird species captured in the Korean peninsula. Prevalence according to year was 68.2% (189 positives/277 tested; 95% CI, 62.4~73.7%) for 2007, 86.4% (367/425; 95% CI, 82.7~89.5%) for 2008, and 95.3% (585/614; 95% CI, 93.3~96.8%) for 2009 (Fig. 2). The regional seroprevalence of positive HI titers ≥ 1 : 20 ranged from 82.9 to 100%. Compared to other provinces, the Gyeongsangnam-do showed the highest prevalence (100%); however, no significant difference in regional prevalence was found. The regional seroprevalences were 100% (80/80) in Gyeongsangnam-do, 93.0% (80/86; 95% CI, 85.4~97.4%) in Jeollanam-do, 91.1% (216/237; 95% CI, 86.8~94.4%) in Jeollabuk-do, 84.7% (210/248; 95% CI, 79.6~88.9%) in Gyeonggi-do, 84.6% (176/208; 95% CI, 79.0~89.2%) in Chungcheongbuk-do, and 82.9% (379/457; 95% CI, 79.2~86.3%) in Chungcheongnam-do. The distribution of JEV seropositive wild bird species ranged from 78.6 to 100%. Seroprevalence according to wild bird species were 100% (4/4; 7/7; 89/89) in Oceanodroma castro, Anas formosa, and Anas acuta (winter visitor); 96.7% (29/30; 95% CI, 82.8~99.9%) in Fulica atra, 90.8% (443/488; 95% CI, 87.9~93.2%) in Anas poecilorhyncha (migratory breeder), 87.9% (188/214; 95% CI, 82.7~91.9%) in Anas platyrhynchos, 80% (16/20; 95% CI, 56.3~94.3%) in Anas penelope (winter visitor), 78.7% in Aix galericulata (migratory breeder), and 78.6% (121/154; 95% CI, 71.2~84.8%) in Anas crecca. The prevalence of wild birds with an HI antibody titer above 1 : 20 or 1 : 1,280 were 86.7% and 21.2%, respectively. Wild birds captured in 2009 had a higher JEV seropositive rate than those captured in 2007 (Fig. 2). Among the wild birds captured in 2009 that had an HI antibody titer of 1 : 20 or more, the most frequent HI titer was 1 : 640 (16.2%).
Climate change, global warming, environmental destruction, and rapid urbanization can alter wild bird migratory paths and increase the incidence of arboviral disease outbreaks. Under these conditions, vector-borne diseases caused by JEV, WNV, and TBEV may be easily introduced into disease-free countries by migratory wild birds. Since JEV was first isolated in Korea in 1946 [9], several Korean JEV isolates have been reported and characterized based on a C/prM and E gene nucleotide analysis. Phylogenetic analyses to determine the genetic relationships between JEV isolates found that Korean JEV isolates changed from genotype 3 to genotype 1 around 1993 [21]. However, few studies have addressed the possibility of JEV introduction into Korea via wild birds. Thus, we conducted a nationwide survey of JEV antibodies in wild birds, including migratory birds.
In this study, the sera of wild birds that were captured in 16 locations of the Korean peninsula were tested for the presence of JEV antibodies. The overall JEV seropositive rate was 86.7%. This HI test result was surprisingly high and suggested that most of the wild birds we captured in Korea had been exposed to JEV. Saito et al. [12] reported that 85.9% and 5.4% of wild ducks captured in Hokkaido, Japan have antibodies against JEV according to the focus-reduction neutralization test (FRNT50 and FRNT90), which is more specific than the HI test. Additionally, 7.2%, 20.4%, and 28.1% of wild birds in 2007, 2008, and 2009, respectively, had a titer > 1 : 1,280. Sugiura and Shimada [15] reported that horses experimentally inoculated with JEV had an antibody titer of 1 : 1,280 within 2 or 3 weeks after inoculation. Therefore, wild birds with titers of 1 : 1,280 or higher may have been infected with wild JEV within 3 weeks. Although we conducted our serosurveillance study with an HI test, which is known to be less specific than the FRNT, our survey results correspond well with those of a previous study in wild ducks in Japan [12]. However, cross-reaction between JEV and WNV in serodiagnoses is a well-known occurrence in Flavivirus-infected animals, including birds [4,6]. Therefore, it is necessary to perform a more specific method, such as a plaque-reduction neutralization test (PRNT), for JEV serosurveillance in birds.
Several positive JEV serosurveillance results have been reported in different animals including swine, goats, cattle, horses, and wild boars in Japan and Korea [16]. It has been suggested that goats may serve as good sentinel animals for serological monitoring of JEV infection because they are currently not vaccinated against JEV in Korea [21,22]. Unlike domestic animals, wild birds cannot be inoculated with a JEV vaccine, but they are easily exposed to several kinds of arthropods such as mosquitoes, ticks, and midges. The high number of positive JEV titers in this study does not indicate viral intrusion into Korea since JEV viremia ends when JEV-specific antibodies are detected in ducks [3]. However, wild birds with viremia may carry JEV to several countries.
According to data obtained from the Korean Center for Disease Control and Prevention, 45 JEV cases in human were reported between 2007 and 2010. More than half of these cases occurred in 2010, suggesting that climate change or a high JEV infection rate among wild birds may, in part, explain the increasing JEV infection rate in the human population. Gyeonggi-do had a high incidence rate (42.3%) of JEV infection in humans, but no significant relationship was found between the seropositive rates in the capture areas and the JEV incidence rate in humans. Nevertheless, new JEV genotypes may be introduced into Korea via wild or migratory birds. It is therefore necessary to monitor JEV antibodies using the PRNT in wild birds. Taken together, these findings lead us to predict that JEV infections may appear in domestic animals such as pigs.
In conclusion, the results of the present study suggested that wild birds with a high incidence rate (86.7%) of JEV antibodies may be introducing new JEV genotypes into Korea. Further studies are needed to isolate all JEV genotypes from blood obtained from migratory birds. Various research projects examining disease distribution and transmission associated with climate change in the Korean peninsula should also be carried out in the near future.
Figures and Tables
Fig. 1
Frequency of the hemagglutination inhibition (HI) titer distribution among wild birds which were positive for Japanese encephalitis virus (JEV) between 2007 and 2009.
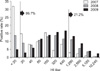
Table 1
Distribution of hemagglutination inhibition (HI) antibody titers against Japanese encephalitis virus (JEV) according to wild bird species

Table 3
Number of JEV cases from 2007 to 2010 in humans residing in Korea

Data were obtained from Korea Center for Disease Control and Prevention (www.cdc.go.kr).
Acknowledgments
The authors would like to thank Ms. JS Kang for providing technical assistance. This work was supported financially by a grant (BAD14-2010-11-03) from the Animal and Plant and Fisheries Quarantine and Inspection Agency (QIA); Ministry for Food, Agriculture, Forestry and Fisheries (MIFAFF), Korea.
References
1. Chen WR, Tesh RB, Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990. 71(Pt 12):2915–2922.

2. Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958. 7:561–573.

3. Dhanda V, Banerjee K, Deshmukh PK, Ilkal MA. Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in domestic ducks. Indian J Med Res. 1977. 66:881–888.
4. Goverdhan MK, Kulkarni AB, Gupta AK, Tupe CD, Rodrigues JJ. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992. 36:277–283.
5. Griffin DE. Emergence and re-emergence of viral diseases of the central nervous system. Prog Neurobiol. 2010. 91:95–101.

6. Hirota J, Nishi H, Matsuda H, Tsunemitsu H, Shimiz S. Cross-reactivity of Japanese encephalitis virus-vaccinated horse sera in serodiagnosis of West Nile virus. J Vet Med Sci. 2010. 72:369–372.

7. Huong VTQ, Ha DQ, Deubel V. Genetic study of Japanese encephalitis viruses from Vietnam. Am J Trop Med Hyg. 1993. 49:538–544.

8. Konishi E, Shoda M, Ajiro N, Kondo T. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J Clin Microbiol. 2004. 42:5087–5093.

9. Kwon HJ, Jang BJ, Lim YM, Lee CK, Jeon YS. Studies on Japanese encephalitis live vaccine. VII. Pathogenicity and immunogenicity of horses with Anyang strain of attenuated virus. Res Rep Natl Inst Vet Res. 1978. 20:29–34.
10. Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004. 10:12 Suppl. S98–S109.

11. Morita K. Molecular epidemiology of Japanese encephalitis in East Asia. Vaccine. 2009. 27:7131–7132.

12. Saito M, Osa Y, Asakawa M. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: risk assessment of invasive flaviviruses. Vector Borne Zoonotic Dis. 2009. 9:253–258.

13. Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ, Barrett ADT. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003. 77:3091–3098.

14. Sucharit S, Surathin K, Shrestha SR. Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. Southeast Asian J Trop Med Public Health. 1989. 20:611–621.
15. Sugiura T, Shimada K. Seroepizootiological survey of Japanese encephalitis virus and Getah virus in regional horse race tracks from 1991 to 1997 in Japan. J Vet Med Sci. 1999. 61:877–881.

16. Sugiyama I, Shimizu E, Nogami S, Suzuki K, Miura Y, Sentsui H. Serological survey of arthropod-borne viruses among wild boars in Japan. J Vet Med Sci. 2009. 71:1059–1061.

17. Takhampunya R, Kim HC, Tippayachai B, Kengluecha A, Klein TA, Lee WJ, Grieco J, Evans BP. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J. 2011. 8:449.

18. van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009. 54:17–35.

19. Wang HY, Takasaki T, Fu SH, Sun XH, Zhang HL, Wang ZX, Hao ZY, Zhang JK, Tang Q, Kotaki A, Tajima S, Liang XF, Yang WZ, Kurane I, Liang GD. Molecular epidemiological analysis of Japanese encephalitis virus in China. J Gen Virol. 2007. 88(Pt 3):885–894.

20. Williams DT, Wang LF, Daniels PW, Mackenzie JS. Molecular characterization of the first Australian isolate of Japanese encephalitis virus, the FU strain. J Gen Virol. 2000. 81(Pt 10):2471–2480.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download