Introduction
P-glycoprotein (P-gp), encoded by the ABCB1 gene (formerly known as MDR1), is a membrane transport protein in the ATP-binding cassette superfamily [16]. P-gp is normally expressed in various mammalian tissues including the apical border of intestinal epithelial cells, brain capillary endothelial cells, biliary canalicular cells, renal proximal tubular epithelial cells, placenta, and testes [4,9,14,17,21]. P-gp functions as an efflux pump on the cell membrane, and thus protects the cell from potentially toxic xenobiotics [16]. Several polymorphisms of the ABCB1 gene are known to cause deformations and dysfunctions of P-gp in humans [3,6,12,18], while in ivermectin-sensitive Collies, a frame shift mutation has been found which causes a premature stop to P-gp synthesis [7,19].
We examined a Border Collie with depression, hypersalivation, and several other adverse reactions which developed following ivermectin intake for heartworm treatment. None of its family members showed any adverse reactions to ivermectin administration. DNA analysis, however, did not reveal the previously described frame shift mutation in the ivermectin-sensitive Border Collie. For this reason, we examined the full-coding sequence of the ABCB1 gene in the ivermectin-sensitive Border Collie, and compared the sequence to those of its family members and to wild-type Beagles in order to investigate the possibility of different mutations.
Materials and Methods
Peripheral blood samples were collected from the cephalic veins of the ivermectin-sensitive Border Collie and its seven family members. Total RNA was extracted from the peripheral blood using TRIzol reagent (Invitrogen, USA). The total RNA was reverse transcribed into first-strand cDNA using a random hexamer primer and the Superscript first-strand synthesis system of the RT-PCR kit (Invitrogen, USA).
To verify the family relationship between the Border Collies, a 261-bp mitochondrial D-loop region was amplified using the primers L15910 and H16498 as previously described [5]. All PCR products were sequenced using an ABI Prism BigDye terminator cycle sequencing ready reaction kit v.5.1 (PE Applied Biosystems, USA) and the sequences were compared.
We designed primers to amplify the coding sequence of the ABCB1 gene; these were based on the wild-type Beagle ABCB1 gene sequence (GenBank accession number NM_001003215). Each primer was designed to contain overlapping sequences of 100~200 bp at both ends of the PCR products. The conditions of the designed primers were verified using DNAMAN software v.4.16 (Lynnon, Canada). Primer information is listed in Table 1. PCR amplification was performed as follows: one cycle at 94℃ for 5 min; 35 cycles at 94℃ for 30 sec at the primer annealing temperature (55~57℃) for 30 sec, followed by at 72℃ for 1 min. The sizes of the resultant PCR products were analyzed using 2% agarose gel electrophoresis.
All PCR products were sequenced using the ABI Prism BigDye terminator cycle sequencing ready reaction kit v.5.1 (PE Applied Biosystems, USA). Finally, all identified sequences were merged (except the overlapping sequences) and compared using CLC sequence viewer v.4.6.2 (CLC Bio, Denmark).
Results
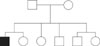
Fig. 1 shows the family pedigree of the Border Collies. All Border Collies except for the father showed the same nucleotide sequence in the 261-bp mitochondrial D-loop region. There was a 99% similarity between the father and the rest of the family.
When compared to the coding sequence of the ABCB1 gene in wild-type Beagle dogs, one insertion mutation and eight single nucleotide polymorphisms (SNPs) were found at the nucleotide level in the ivermectin-sensitive Border Collie (Fig. 2). Three nucleotides ('AAT') were inserted between the 72nd and 73rd nucleotide of the ABCB1 coding sequence. The eight SNPs were characterized as G574A, C635G, A985T, G996A, G1595A, T2082A, T2086C, and A3817G. At the protein level, the insertion mutation led to the addition of an asparagine between the 24th and 25th amino acids. Six of the SNPs lead to amino acid exchanges (Val192Ile, Pro212Arg, Thr329Ser, Arg532Gln, Ser696Pro, and Ile273Val), while G996A and T2082A resulted in silent mutations. All SNPs were also examined in all of the Border Collie family members; however, the insertion mutation was observed only in the ivermectin-sensitive Border Collie.
Discussion
P-gp was first discovered in 1976 in a Chinese hamster ovary (CHO) cell line that was selected in culture for colchicine resistance [10]. Resistant CHO cells expressed large quantities of a 170-kD protein, subsequently named P-gp. Since then, several drugs have been identified as substrates of P-gp [6]. In dogs, ivermectin sensitivity caused by a nonsense mutation of the ABCB1 gene is a well-recognized phenomenon [19]; however, the full sequence of the ABCB1 gene is only available for the wild-type Beagle. Therefore, we first established the ABCB1 coding sequence in wild-type Border Collies and compared that to the wild-type Beagle sequence. We found eight SNPs in all wild-type Border Collies. Taking into account interethnic variation in the human ABCB1 sequence [1,20], our finding suggests that the canine ABCB1 sequence may demonstrate interbreed variation. For this reason, we recommend that the ABCB1 gene sequence be confirmed in each breed of dog prior to molecular investigation for mutations.
Using the wild-type Border Collie sequence examined in this study as a baseline for comparison, we investigated alterations in the sequence of the ABCB1 gene in an ivermectin-sensitive Border Collie that lacked the expected frameshift mutation. While eight SNPs were found in all of the study Border Collies, an insertion mutation was found only in the ivermectin-sensitive animal. The conformational change of P-gp is important for the protein's role as an efflux pump through interaction between the drug-binding and nucleotide-binding domains [15]. In the human ABCB1 gene, several SNPs have been identified that can lead to decreased P-gp function [6]. Furthermore, a silent mutation in the ABCB1 gene has been found to be associated with the level of P-gp expression in humans [8]. Deformation of protein structure caused by the addition or substitution of a single amino acid also induces several diseases in human and animals [2,13]. Malfunction of P-gp has often been noted in Collies and can occur either by decreased expression of a functional gene or by a gene mutation that impairs protein concentration or activity [19]. The eight SNPs identified in this study do not appear to be directly related to the adverse reactions displayed by the ivermectin-sensitive Border Collie, suggesting the possibility that these polymorphisms of the ABCB1 gene are related to the interbreed variation in P-gp function seen in dogs.
Since mitochondrial DNA is inherited exclusively from the mother, it can be used as a tool for tracking maternal lineage [23]. A D-loop-containing region that has a highly variable sequence is commonly used for phylogenetic analysis [11,22,24]. To verify the family relationship of the Border Collies, we sequenced the D-loop region and found that all family members expressed the same sequence except for the father dog. The similarity between the father dog and the rest of the family was 99%. According to the GenBank database, the similarity of the sequence of canine D-loop regions is 98~99%. This suggests that the SNPs of the ABCB1 coding sequence identified in this study are a universal feature of Border Collies.
In conclusion, we found a new insertion mutation of the ABCB1 gene in an ivermectin-sensitive Border Collie. This mutation was not seen in its healthy family members or in the normal Beagle. Additionally, we described eight SNPs in the wild-type Border Collies. This finding suggests the possibility that dogs display interbreed variation in the ABCB1 sequence. The sequence of the ABCB1 gene should be established in each breed of dog prior to molecular investigation of the ABCB1 gene and P-gp.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download