Abstract
Blood, saliva, and nail samples were collected from 54 dogs and 151 cats and analyzed for the presence of Bartonella henselae with a novel nested polymerase chain reaction (PCR) method. Bartonella (B.) henselae was detected in feral cat blood (41.8%), saliva (44.1%), and nail (42.7%) samples. B. henselae was also detected in pet cat blood (33.3%), saliva (43.5%), and nail (29.5%) samples and in pet dog blood (16.6%), saliva (18.5%), and nail (29.6%) samples. Nine samples were infected with B. clarridgeiae and 2 were co-infected with B. henselae and B. clarridgeiae of blood samples of dogs. This report is the first to investigate the prevalence of B. henselae and B. clarridgeiae in dogs and cats in Korea, and suggests that dogs and cats may serve as potential Bartonella reservoirs.
The genus Bartonella (B.) includes at least 20 species and subspecies, and several of these are human pathogens [22]. Clinical manifestations of Bartonella infection include Carrion's disease, trench fever, cat scratch disease, bacillary angiomatosis, endocarditis, chronic bacteremia, neuroreti nitis, and osteomyelitis [13]. Cat scratch disease is zoonotic and primarily caused by B. henselae [15]. B. clarridgeiae can also cause cat scratch fever. B. henselae and B. clarridgeiae can also infect dogs [4,7], and both species can function as bacterial reservoirs for infection [5,9,10,18,19]. Cat scratch disease was recently reported in a woman with a pet dog in Korea [5]. However, the prevalence of Bartonella spp. from companion animals in Korea has not been previously investigated. We examined the prevalence of B. henselae and B. clarridgeiae in dogs and cats in the present study using a recently developed nested PCR method.
Blood, saliva, and nail samples were collected from healthy pet dogs (n = 54) and cats (n = 48) at the Veterinary Medical Teaching Hospital of Seoul National University, Korea. All samples were collected from November 2005 to July 2006. Feral cats (n = 103) were captured in neighborhoods throughout Seoul and were isolated in an animal shelter. B. henselae strain Houston-1 (ATCC 49882) and B. clarridgeiae strain (ATCC 51734) were obtained from the American Type Culture Collection (USA) and used for positive control samples. Genomic DNA was extracted using Genomic Blood DNA and Genomic Cell/Tissue DNA Extraction Kits (iNtRoN Biotechnology, Korea), per the manufacturer's instructions. Primary PCR was performed with the P-bhenfa (5'-TCTTCGTTTCTCT TTCTTCA-3') and P-benr1 (5'-CAAGCGCGCGCTCTA ACC-3') primers which amplified B. henselae (186 bp) and B. clarridgeiae (168 bp) fragments. Nested PCR amplified B. henselae (152 bp) and B. clarridgeiae (134 bp) fragments with the N-bhenf1a (5'-GATGATCCCAAG CCTTCTGGC-3') and N-bhenr (5'-AACCAACTGAGC TACAAGCC-3') primers [15]. Primary and nested PCR reactions were performed as previously described [15].
All PCR products were analyzed by sequencing with an automated sequencer ABI 3100 Genetic Analyzer (Bionics, Korea) and results were confirmed to be from B. henselae (GeneBank access number DQ000494) and from B. clarridgeiae (GeneBank access number: DQ003029).
B. henselae was detected in 14.2% of blood samples (14/98), 3.9% of saliva samples (4/102), and 4.8% of nail samples (5/103) from feral cats. In contrast, only 6.3% (3/48) of blood samples from pet cats were positive for B. henselae. B. henselae was not detected in pet cat saliva samples (n = 46), pet cat nail samples (n = 44), or in any pet dog samples (n = 54).
B. henselae was detected in 41.8% (41/98) of blood samples, 44.1% (45/102) of saliva samples, and 42.7% (44/103) of nail samples from feral cats by nested PCR. In addition, 33.3% of blood samples (16/48), 43.5% of saliva samples (20/46), and 29.5% of nail samples (13/44) from pet cats were B. henselae positive. B. henselae DNA was also detected in 16.6% (9/54) blood samples, 18.5% (10/54) of saliva samples and 29.6% (16/54) of nail samples from dogs (Table 1).
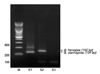
B. clarridgeiae was detected in 2 feral cat blood samples, a feral cat saliva sample, 3 dog blood samples, a dog saliva sample, and 2 dog nail samples. Additionally, 2 samples (1 dog blood and 1 dog nail) were co-infected with B. henselae and B. clarridgeiae (Table 2). PCR product and DNA sequencing data are shown in Fig. 1.
Cats are usually the main zoonotic reservoir for Bartonella infection [14], although dogs may also serve as zoonotic reservoirs secondary to B. henselae and B. clarridgeiae infection [4,7]. Cat scratch disease was identified in a case with suspected human:canine transmission in Korea [5,18]. However, there are no current surveys evaluating Bartonella spp. prevalence in cats and dogs.
A previous study reported that 39% of cats were B. henselae positive among a population of 146 cats in Japan [11], and this result was significantly higher than the previous 7.2% prevalence among cats in Japan. Previous studies conducted in various countries identified higher Bartonella bacteremia prevalence in shelter cats than in pet cats [2,3,8,10]. The Bartonella prevalence in pet cats in the present study (33.3%) was significantly higher than the prevalence in previous studies, including Germany (13%), France (11%), and the Netherlands (22%) [1,5,17]. Conversely, the prevalence of B. henselae in sheltered cats (41.8%) was similar to the prevalence identified in other studies. These findings suggest that pet cats may serve as a reservoir for B. henselae infection to their owners. This is particularly relevant to immunocompromised pet owners.
B. henselae prevalence in cats is higher than B. clarridgeiae prevalence [16], but this may be dependent on age, sex, and type of breeding [6]. The B. henselae prevalence in cats and dogs was greater than B. clarridgeiae and was higher in cats than in dogs. These results supported previous studies which suggested that B. henselae was the major zoonotic pathogen. A recent survey of Bartonella seropositive healthy blood donor in Sweden demonstrated a similar prevalence to dogs in the present study [12]. Zoonotic diseases have become an increasingly important public health concern [5]. Our results suggest that B. henselae and B. clarridgeiae are highly prevalent in Korean cats and dogs. Further, cats and dogs may serve as reservoirs for human Bartonella infection.
In conclusion, data from the present study suggests that Bartonella infection prevalence in Korean shelter cats is similar to those of previously described countries. However, the prevalence of B. henselae in Korean pet cats was higher than reported prevalence in other countries. This is the first report examining the prevalence of B. henselae and B. clarridgeiae infection in domestic cats and dogs in Korea.
Figures and Tables
Fig. 1
Bartonella (B.) henselae (S2) and B. clarridgeiae (S1) nested PCR amplification bands from 2 cats. The negative control band (S3) is visualized on the right side. M: standard maker.
Acknowledgments
This work was supported by Korean Research Foundation Grant (KRF-2006-005-J02902). Further supports were also provided by the Research Institute of Veterinary Science, and the BK21 Program for Veterinary Science, College of Veterinary Medicine, Seoul National University.
References
1. Bergmans AM, de Jong CM, van Amerongen G, Schot CS, Schouls LM. Prevalence of Bartonella species in domestic cats in The Netherlands. J Clin Microbiol. 1997. 35:2256–2261.

2. Branley J, Wolfson C, Waters P, Gottlieb T, Bradbury R. Prevalence of Bartonella henselae bacteremia, the causative agent of cat scratch disease, in an Australian cat population. Pathology. 1996. 28:262–265.

3. Chomel BB, Carlos ET, Kasten RW, Yamamoto K, Chang CC, Carlos RS, Abenes MV, Pajares CM. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines. Am J Trop Med Hyg. 1999. 60:593–597.

4. Chomel BB, Mac Donald KA, Kasten RW, Chang CC, Wey AC, Foley JE, Thomas WP, Kittleson MD. Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol. 2001. 39:3548–3554.

5. Chung JY, Han TH, Kim BN, Yoo YS, Lim SJ. Detection of Bartonella henselae DNA by polymerase chain reaction in a patient with cat scratch disease: a case report. J Korean Med Sci. 2005. 20:888–891.

6. Engvall EO, Brändström B, Fermér C, Blomqvist G, Englund L. Prevalence of Bartonella henselae in young, healthy cats in Sweden. Vet Rec. 2003. 152:366–369.
7. Gundi VA, Bourry O, Davous B, Raoult D, La Scola B. Bartonella clarridgeiae and B. henselae in dogs, Gabon. Emerg Infect Dis. 2004. 10:2261–2262.
8. Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997. 35:1327–1331.

9. Keret D, Giladi M, Kletter Y, Wientroub S. Cat-scratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br. 1998. 80:766–767.

10. Marston EL, Finkel B, Regnery RL, Winoto IL, Graham RR, Wignal S, Simanjuntak G, Olson JG. Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin Diagn Lab Immunol. 1999. 6:41–44.

11. Maruyama S, Nakamura Y, Kabeya H, Tanaka S, Sakai T, Katsube Y. Prevalence of Bartonella henselae, Bartonella clarridgeiae and the 16S rRNA gene types of Bartonella henselae among pet cats in Japan. J Vet Med Sci. 2000. 62:273–279.

12. McGill S, Wesslén L, Hjelm E, Holmberg M, Auvinen MK, Berggren K, Grandin-Jarl B, Johnson U, Wikström S, Friman G. Bartonella spp. seroprevalence in healthy Swedish blood donors. Scand J Infect Dis. 2005. 37:723–730.
13. Molia S, Chomel BB, Kasten RW, Leutenegger CM, Steele BR, Marker L, Martenson JS, Keet DF, Bengis RG, Peterson RP, Munson L, O'Brien SJ. Prevalence of Bartonella infection in wild African lions (Panthera leo) and cheetahs (Acinonyx jubatus). Vet Microbiol. 2004. 100:31–41.

14. Pons I, Sanfeliu I, Quesada M, Anton E, Sampere M, Font B, Pla J, Segura F. Prevalence of Bartonella henselae in cats in Catalonia, Spain. Am J Trop Med Hyg. 2005. 72:453–457.
15. Rampersad JN, Watkins JD, Samlal MS, Deonanan R, Ramsubeik S, Ammons DR. A nested-PCR with an Internal Amplification Control for the detection and differentiation of Bartonella henselae and B. clarridgeiae: an examination of cats in Trinidad. BMC Infect Dis. 2005. 5:63.
16. Rolain JM, Locatelli C, Chabanne L, Davoust B, Raoult D. Prevalence of Bartonella clarridgeiae and Bartonella henselae in domestic cats from France and detection of the organisms in erythrocytes by immunofluorescence. Clin Diagn Lab Immunol. 2004. 11:423–425.

17. Sander A, Bühler C, Pelz K, von Cramm E, Bredt W. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J Clin Microbiol. 1997. 35:584–587.

18. Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998. 352:1682.
19. Windsor JJ. Cat-scratch disease: epidemiology, aetiology and treatment. Br J Biomed Sci. 2001. 58:101–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download