Introduction
Although bone tissues are capable of regenerative growth, the repair process is inadequate in many clinical and pathologic situations, including massive bone loss caused by trauma and tumor resection, as well as the reconstructive surgery required to correct developmental deformities. The lost bone can be replaced by endogenous or exogenous bone tissues, which is associated with several disadvantages. The properties required for ideal bone substitutes include biocompatibility, biodegradability, ability to provide structural support, capacity to serve as drug carriers, ease of use in clinical practice, and an affordable cost/benefit ratio [8,22,31]. Due to the limited availability and donor site morbidity of bone autografts, and the risk of possible immune responses, disease transmission, and the cost of allografts, the use of synthetic bioactive materials opens new possibilities for clinical application, mainly in orthopaedics and dentistry [5,31,38,42].
A number of materials, such as metals, metal alloys, collagen, carbon-based materials, polymers, ceramics, and composites of the above materials have been recommended to fill and reconstruct bone defects, but none have been shown to be ideal. However, metals are being widely used for major load-bearing orthopedic applications [28]. The materials have many limitations, though, due to unfavourable corrosion properties, wear, encapsulation by dense fibrous tissues to develop improper stress distribution, and/or adverse tissue reactions [17]. Several non-metallic materials have been proposed for reconstruction of bone, but none have been found to be suitable for wide application in clinical conditions. Biocompatibility, along with biodegradability and suitable mechanical properties of materials, are essential prerequisites for mimicking natural bone, which unfortunately exists in a small group of materials. Although autogenous bone grafts are still considered the gold standard for bone replacement, and allogenic bone grafts are widely used, several ceramic biomaterials have been developed as synthetic bone substitutes, thus challenging their supremacy.
For the current study, calcium-phosphate ceramics, such as hydroxyapatite (HAp), have been used because their chemical composition is closely related to that of the mineral phase of bone [19]. These ceramics are adequately biocompatible [10] and do not induce adverse local tissue reactions, immunogenicity, or systemic toxicity. Furthermore, because this material is osteoconductive, it acts as a support for new bone formation within the pore sites [24], which are deliberately generated in the structure. However, depending on the preparation technique, the material exhibits gross different powder characteristics, microstructure, and associated mechanical and biologic properties. When nano-sized particles below 100 nm of HAp are concerned, it is still a challenge to synthesize the same via a simple method. Moreover, for repair and reconstruction of diseased or damaged bones or tissues, a biphasic calcium phosphate (BCP) composed of a suitable percentage of HAp and β-tri-calcium phosphate (β-TCP) are thought to be near the ideal solution for this remodeling of bone. The first studies of LeGeross et al. [23] on BCP with varying HAp/β-TCP demonstrated that the bioactivity of these ceramics may be controlled by manipulating the HAp/β-TCP ratios. Although various routes have been developed to synthesize HAp powders [16], only a few reports are available concerning the production of β-TCP [20]. For the synthesis of both materials, the most commonly adapted technique is wet chemical precipitation [2], followed by calcinations. We have successfully synthesized a series of BCP composition with varied HAp and β-TCP content by using a novel aqueous combustion technique. This processing technique is often adapted for the rapid preparation of a variety of oxide ceramic powders [20]. The process involves an exothermic, usually very rapid and self-sustaining chemical reaction between the desired metal salts (oxidizer), preferably nitrates, and a suitable organic fuel, such as urea, glycine, carbohydrazide, and citric acidin an aqueous solution. The reaction is initiated at a fairly low temperature followed by rapid cooling, and this in turn leads to nucleation of crystallites without much growth. The reaction between the oxidizer and fuel releases large amounts of reaction heat that is utilized to synthesize the desired materials in situ and the large volume of gas evolved disintegrates the high purity products to friable agglomerates of very fine particulates.
The purpose of the present study was to evaluate porous HAp, the powders of which were prepared by a novel aqueous solution combustion technique, as a bone substitute in healing bone defects.
Materials and Methods
Synthesis of nano-crystalline HAp by an aqueous solution combustion method
Calcium nitrate tetrahydrate (S.D. Fine-Chem, India) and di-ammonium hydrogen ortho-phosphate (DAP; S.D. Fine-Chem, India) were used as raw materials for the preparation of calcium phosphate powders. Urea (Glaxo, India) and glycine (Glaxo, India), both A.R. grade, were used as the fuel. For synthesizing HAp, aqueous stock solutions of calcium nitrate tetrahydrate (2.72 M) and DAP (2.09 M) were first mixed slowly with continuous stirring; subsequently concentrated nitric acid was added dropwise to dissolve the resulting white precipitate. A predetermined amount of solid fuel was added to the clear solution and homogenized by stirring with a magnetic stirrer for 30 min. at room temperature. One glass ceramic-coated mild steel (dia. ~80 mm, volume 130 ml) container containing the solution was introduced into a muffle furnace preheated to the desired temperature (300-700℃). A stainless steel wire mesh was put on the reaction container to reduce particle loss through aerosol formation. Immediately after placement in the furnace, the mixed solution started to boil, followed by the evolution of a large volume of gases. The mass then frothed and swelled to yield foam, from where a flame appeared and burned with incandescence. At the initiation of ignition, the furnace was switched off. The heat evolved during the reaction sustained itself and proceed to completion without requiring any further heat from an external source. The general flowchart for the process is shown in Fig. 1. Details of the in vitro characterization of the prepared powder are beyond the scope of this article, but can be found elsewhere [12]. This powder has been used for the following studies.
Fabrication of porous HAp
In the present study, porous (35-40% by volume) HAp was fabricated by using β-naphthalene and polyvinyl alcohol (S.D. Fine-Chem, India) as a combustible organic material. HAp powder was milled separately with oleic acid surfactant and a pre-calculated amount of β-naphthalene. Rectangular-shaped (12 × 5 × 3 mm3) blocks were uniaxially cold-compacted with low pressure, and subsequently cold iso-statically pressed at 100 MPa for homogeneous densification. All specimens were slowly dried at 80℃ for 3 days. Finally, HAp specimens were sintered at 1,250℃ for 2 h. Archimedes' principle using water as the immersing medium was used to calculate the density and apparent porosity of the sintered specimens. Scanning electron microscopy (SEM) and mercury intrusion porosimetry (MIP) were used to obtain the pore shape, size, morphology, and distribution of the specimens. Fig. 2A shows the SEM photomicrograph of the porous strut with a tag of 5 µm, while Fig. 2B shows the histogram based on the MIP data for distribution of the pores in the struts. The porous struts were initially pasteurized with distilled water and subsequently autoclaved at 121℃ for 30 min. before implantation. Archimedes' principle using water as the immersing medium was used to calculate the density and apparent porosity of the sintered specimens and found to be 2.04 g/ml and 35.2% on an average, respectively.
Animal experimentation
Animal experimentation was carried out following the procedures conforming to the standards of the Institutional Animal Ethical Committee of the West Bengal University of Animal and Fishery Sciences. Twelve black Bengal goats of both genders, weighing 10-12 kg, were randomly distributed into 2 groups of 6 animals each, as follows: control (group I), in which the bone defect was not treated and the test specimen (group II), in which porous HAp blocks were inserted within the bone defect. Under standard aseptic conditions and sedation with xylazine hydrochloride (0.05 mg/kg body weight; Indian Immunologicals, India) in animals which had received atropine and local 2% lignocaine hydrochloride (Neon Laboratories, India), a 3 cm longitudinal skin incision was made on the lateral side of the radius bone. The implant sites (1 × 0.5 cm) were prepared using a micro-motor dental drill after exposing the cortical bone followed by irrigation with sterile normal saline. In the controls (group I), the defect was left as such without any implant, while in group II, HAp blocks were placed in the defect sites. The implants were secured in position by suturing the periosteum, muscle, subcutaneous tissue, and skin in layers. Postoperatively, all the animals received cefotaxime sodium (250 mg I/M twice daily; Mapra India, India) and injectable meloxicam (0.5 ml once daily for 5 days; Intas Pharmaceuticals, India) with daily dressing changes of the surgical wounds.
Local inflammatory reaction and healing of the wound
Local inflammatory reactions and healing of the wounds were assessed by visual and manual examinations from the day of surgery up until the 90th day postoperatively.
Radiological examination
Radiographs were obtained of the operated forelimb immediately after implantation and subsequently on days 21, 30, 60, and 90 postoperatively to assess the status of the implant, the host-bone reaction to the implant, and new bone formation. X-rays were also obtained after light sedation using xylazine hydrochloride (0.05 mg/kg body weight).
Histological study
The implanted ceramic blocks, along with the surrounding bones, were collected from the animals on day 90 postoperatively. The bone sections with both normal and implanted areas were prepared by decalcification following a standard technique; 4 µm sections were cut and stained with hematoxylin and eosin to observe the status of the bone implants and the cellular response of host bone to the implants.
Oxytetracycline labeling study
Fluorochrome (oxytetracycline dehydrate; Pfizer India, India), at a dose of 50 mg/kg body weight, was given on days 77, 78, 85, and 86 (2-6-2 i.e. two injections on day 77 and 78 and after 6 days another two injections on days 85 and 86) post-operatively for double-toning of new bone. Undecalcified ground sections were prepared [27] from the implanted segments of bone and the sections were ground to 20 µm thickness using different grades of sand paper. The ground-undecalcified sections were observed under ultraviolet incidental light with an Orthoplan microscope (Excitation filter, BP-400 range; Leitz, USA) for tetracycline labeling to determine the amount and source of newly formed bone.
Angiographic study
Radial angiography was performed by making a 4-5 cm skin incision aseptically on the medial aspect of the thigh under xylazine hydrochloride sedation and local infiltration analgesia with 2% lignocaine hydrochloride on day 90 postoperatively. The radial arteries were located, exteriorized, and catheterized using polyethylene catheters connected to a syringe containing 15 ml sodium iothalmate (Mallinckradt, USA). The contrast material was infused with regular gentle digital pressure and radiographs were taken at 14 mAs, 50 kVP, and 90 cm FFD. The catheter was removed and the puncture of the artery was sutured with 4-0 chromic catgut, and finally the skin wound was closed. For better visualization of the arteries, one test limb from each group was collected after euthanizing the animal at the end of the experiment; the limb was perfused with lead oxide suspension (20% W/V) in a manner similar to that used to examine the vascular response of host bone and surrounding tissues in the implanted area and visualization of the implant.
Results
Local inflammatory reactions and healing of the wound
No marked inflammatory reactions were observed in the control and experimental groups following placement of bioceramic implants up to the 90th day postoperatively. Weight-bearing capacity in each animal gradually improved, as signs of inflammation subsided (within 10 days). There was no adverse local effect, such as marked hematoma or edema, during the early postoperative period. Wound healing was uneventful in all cases and the sutures were removed on the 10th postoperative day. The implants were clinically stable in the bone.
Radiological observations
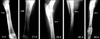
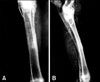
On day 0 in group I (control), the radiographs showed the cortical defect devoid of any implant, resulting in a radiolucent gap. On day 21, the radiographs revealed a minimal periosteal reaction and smoothing edges with oval-shaped corners of the cortical bone defects. On day 30, radiographs indicated a substantial reduction in the gap size, which was in the process of obliteration by hard tissue materials of similar density to that of host bone. On day 60, the defect was not totally obliterated by newly grown bony tissue. On day 90, radiographs showed that the defect was similar to what was observed after day 60, except that the newly formed bony tissue was more organized and the fractured end became smooth and round. Representative radiographs are shown in Fig. 3.
Radiographs obtained on day 0 of group II (HAp) of the defect site showed a rectangular-shape mid-shaft diaphyseal defect with a well-placed HAp block and a radio-density of the implant, similar to that of the host bone. On day 21, the diagram showed a well-established periosteal reaction with narrowing of the gap between the bone and implant without any signs of implant resorption. On day 30, the radiographs showed the presence of the implant and radiologically-detectable newly grown host tissue. On day 60, the implant was noted to have a reduced density in comparison to the radiographs of previous days. On day 90, there was complete bridging of the cortical defect along the axis of the radius with a similar radio-dense bony material to that of normal bone. The presence of the implant could be identified by a radio-dense shadow in the implanted site and the implant was not absorbed, rather it had undergone structural changes by a host graft interaction. Representative radiographs are shown in Fig. 4.
Histological study
Tissue sections from group I (control) showed mild inflammatory reactions with moderate fibro-collagenization. The cortex showed a lamellar appearance of the bone along with the presence of woven bone in some places. The marrow space showed an adequate amount of marrow material, fat cells, and blood vessels (Figs. 5A and B).
Tissue sections of group II (HAp) showed complete normal ossification with development of Haversian canals and well-defined osteoblasts at the periphery. The blood vessels in the Haversian spaces were well-developed. The marrow space showed development of blood vessels with very little amount of marrow material. Non-absorbed biodegradable material was also noted in the lamellar cortical bone and in the marrow space as a refractile, crystalloid structure (Figs. 6A and B).
Oxytetracycline labeling study
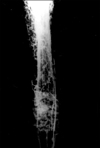
In group I (control), the process of new bone formation was active from both ends. Newly formed osseous tissues originating from the periosteal, as well as the endosteal, surface of the bone were seen, however, the intensity was dominant on the periosteal side. The defect was completely filled with newly formed cancellous bone and appeared as a homogenous non-fluorescent area. However, a narrow linear zone near the periosteum revealed a golden-yellow fluorescence, suggestive of new bone formation in the area (Fig. 7). Union in the defect site of the bone was complete in most of the animals.
In group II (HAp) under fluorescent microscopy, the defect line was visualized as a line of golden-yellow fluorescence, whereas the host bone evinced a dark, sea green homogenous colour. In this group, the activity of new bone formation was moderate. Within this new osteoid tissue, which completely filled the bone defect; crossing-over of the new bone trabeculae was evident. Resorption cavities were present, indicating that the resorption and replacement of bone were well under progress (Fig. 8).
Angiographic study
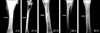
Angiograms of the animals in group I (control) showed that there was uniform angiogenesis in the defect site. Establishment of trans-transplant angiogenesis was evidenced by the presence of a capillary network on and around the defect site containing radio-dense contrast material. The angiogram also revealed the establishment of a uniform medullary cavity (Fig. 9).
Angiograms of group II (HAp) on day 90 postoperatively revealed the presence of intact radio-dense transplant material with a slight alteration of shape and size. There was completion of trans-transplant shunting of blood vessel communication which was well-depicted due to the presence of the positive contrast used during angiography. The lateral radiograph of the same animal also revealed that there were well-established, regularly arranged blood vessels in the periphery of the transplant (Fig. 10).
Discussion
Bone grafts are often necessary to provide support, fill voids, and enhance biologic repair of skeletal defects. Strategies for the development of biologic substitutes capable of mimicking the natural environment aim to provide the key components which play a pivotal role in the repair of the bone [6,40]. Autogenous bone is the gold standard that all alternatives must meet or exceed. However, autografts have significant limitations; including donor site morbidity, inadequate availability, and inappropriate form [3,9,36]. These limitations have prompted increasing interest in alternative bone grafts. In recent years, considerable strides have been made with the use of ceramics/polymers in orthopedic surgery, particularly as permanent implants or joint replacement. The incorporation of these materials in host bone is clearly inferior to autogenous bone grafts. They enhance osteoconduction, which is a three-dimensional process of the growth of the capillaries, perivascular tissue, and osteoprogenitor cells of the host into the graft [15].
In the present study, clinical signs were of little importance in evaluating the process of healing after reconstruction of bone defects by different types of implants. However, the type of wound healing and restoration of function provided a rough idea about the status of soft tissue and bone healing. In all the surgically-created defect areas, the implants were well-placed, well-accepted, and tolerated by the animals, causing no significant inflammation in the surrounding tissues. Healing was uneventful in all animals and there was no evidence of rejection of the implant in any case. The clinical features of the present study corroborated the findings of Holmes et al. [18]. Lameness gradually resolved, suggesting that the inflammation had subsided and the fracture was stabilizing, which corroborated the findings of ulnar fractures in dogs [32] and rabbits [35]. No foreign body response or toxicity was elicited and hence all the implants were accepted as a suitable alternative bone graft to fill the defect.
Critical evaluation of radiographs taken at different intervals in the animals of group I revealed moderate evidence of fracture union as compared to the other group. However, in the initial stages, minimal periosteal reactions and smoothing edges of the cortical bone defects were noticed. This may be due to the larger defect size, which is in agreement with the observations of Singh [35]. Subsequently, there was a substantial reduction of gap size by newly formed osseous tissue, making the defect more round and smooth. A similar finding has also been reported by other workers [4].
In the animals of group II, day 0 radiographs revealed the presence of well-placed HAp blocks in the mid-shaft radial diaphyseal defects which were indistinguishable to the radio-density of host bone [35]. On day 21, there were well-established periosteal reactions without any signs of implant resorption. On day 30, the HAp implants were in the process of resorption from all four corners and the HAp implants were replaced by radiologically-detectable newly grown bone, which is in agreement with other observations [35]. On day 60, the cortex of the defect along the longitudinal axis was bridged with newly formed bony tissue, indicating a well-organized healing process. Complete bridging of the cortical defect was observed on day 90 with similar radio-dense bony material and the implant was encapsulated. It has been reported that 52.7% of the bone defect is replaced by lamellar bone and 27.5% of the HAp implant degraded within 24 weeks [30]. With the increase of pore size, the rate of resorption and replacement of this implant by the new bone also increased [11]. Besides, a change in surface area seemed to be the greatest factor affecting the rate of resorption of HAp ceramics [29]. The present results indicate that the pore size of the HAp implant was not optimum for ingrowths of new bone and hence the indication of slow resorption.
In group I, there was moderate fibro-collagenisation with the presence of woven bone in some places. The new bone formation was not sufficient to fill the entire defect, although marrow space showed an adequate amount of marrow materials and blood vessels, which supported the findings of other workers [4,14,35]. However, this was in contrast with the observations of Bolander and Balian [4], who reported that ungrafted ulnae did not successfully heal across the defect and there was a limited amount of new bone formation in the vicinity of the cut end of the defect.
In group II, the bone defect was almost repaired with newly formed osteoid tissue with well-developed blood vessels in Haversian canals and a very small amount of marrow materials sparingly appeared at places, which corroborated the findings of other observations where Hap was implanted in the skulls of rats and skulls and ulnae of rabbits [35,39]. In these cases, the porous HAp acted as a scaffold for the in-growth of vessels and subsequent deposition of new bone, which is in agreement with the observation of Simmons [33] and Alexander et al. [1].
There are several methods to examine newly formed bone using specific bone markers and labeling techniques [21]. The tetracycline labeling method was introduced to measure the exact quantity of newly formed bone as the tetracycline molecule has a fluorescence property in ultraviolet light. Oxytetracycline is absorbed to the areas where active deposition of mineralized tissue is taking place [13]. The labeled new bone and old bone emit bright golden-yellow and dark, sea green fluorescence, respectively, when viewed under UV light and this provides useful information in assessing the amount of new bone formation and fracture healing [25]. In the present study, oxytetracycline labelling (50 mg/kg body weight; a 2-6-2 pattern) before the end of the study was sufficient to quantify the extent of new bone formation at the implanted site of bone.
In animals of group I, most of the bone defects were occupied by a homogenous, non-fluoroscent area, indicating little new-bone formation, although the site and the process of new bone formation was active from both ends [35]. Newly formed bony tissue originated more from the periostial surface as compared to the endosteal side, indicating bony union at the defect site and suggesting a normal healing process. These findings simulated the observation in which two anabolic hormones were used in tibial fracture healing in dogs [37]. However, golden-yellow fluorescence was seen in a narrow linear zone near the periosteum, suggesting new bone formation in the area.
The oxytetracycline labeling study demonstrated that new bone formation in the defect site was greater in group II compared to group I animals. There was little indication of implant contribution towards new bone formation, rather a contribution mainly by the host bone, which is in agreement with the observations of other workers [35]. Resorption cavities were present in Hap-implanted bone, suggesting that the resorption, remodeling, and replacement of the bone were well underway.
Sodium iothalmate, as a contrast media, has been successfully used for the visualization of different vascular patterns [41]. Lead oxide soap suspension (20%), as a contrast media, was found satisfactory for the visualization of major arteries and also minute vascular branches [26]. This material is toxic, cannot be drained out by the venous system, and as such, the animal must be sacrificed before performing angiography.
Critical evaluation of angiographic results of the present study revealed varying degrees of vascularization. However, the evidence of trans-transplant angiogenesis was more pronounced in animals implanted with HAp than the controls. The presence of intact transplant material that was detected by angiography could be attributed to the fact that the transplant was biocompatible and subsequently had low or no inflammatory response, which was also observed by Singh [34]. The minute vessels of periosteal and endosteal origin invadidng the implant bed supports the view that vascularization in fracture healing is directly related to the amount of new bone formation [7]. Angiograms of control animals revealed comparatively less uniform transtransplant angiogenesis, although the medullary cavity was well-established.
In conclusion, nano-HAp powders prepared by a novel aqueous solution combustion technique, is a very simple method by which not only the powder size, but the composition can also be varied for optimum osteoinductive and conductive responses in vivo. Porous HAp ceramic material promoted bone formation over the defect, conforming their biologic osteoconductive property. No inflammatory reaction was observed due to the presence of the implanted material. This has been interpreted and subsequently verified from the experimental results in terms of radiologic and histopathologic methods, angiography, as well as the oxytetracycline labeling studies.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download