Abstract
Although disease-free survival remains the primary goal of prostate cancer treatment, erectile dysfunction (ED) remains a common complication that affects the quality of life. Even though several preventive and therapeutic strategies are available for ED after radical prostatectomy (RP), no specific recommendations have been made on the optimal rehabilitation or treatment strategy. Several treatment options are available, including phosphodiesterase-5 inhibitors, vacuum erection devices, intracavernosal or intraurethral prostaglandin injections, and penile prostheses. Urologists must consider more effective ways to establish optimal treatments for ED after RP. ED is an important issue among patients with prostate cancer, and many patients hope for early ED recovery after surgery. This review highlights the currently available treatment options for ED after RP and discusses the limitations of each.
Radical prostatectomy (RP) is a commonly used treatment option for complete remission of localized prostate cancer. Unfortunately, the operation carries a risk of postoperative complications including erectile dysfunction (ED) [1]. Although great advances have been made in surgical techniques and devices, the prevalence of ED after RP remains a major postoperative complication [2,3].
Owing to the relatively favorable outcome of prostate cancer after RP for localized prostate cancer, patients with prostate cancer not only must live with the constant fear of possible future problems including cancer recurrence or progression but also are faced with significant physical, cognitive, sexual, and socioeconomic problems after treatment [4,5]. Among the postoperative complications, many patients face the distress of sexual difficulties such as loss of erectile function and, in some cases, pain related to sexual activity [2]. Functional outcomes are not always optimal despite increased surgical precision and advanced techniques. A recent meta-analysis found that new robotic surgical techniques do not improve ED after RP [6]. This result has led to the development of several penile rehabilitation programs.
Male sexual dysfunction related to prostate cancer treatment can be divided into three broad categories: (1) ED and changes in penile size and shape, (2) ejaculatory and orgasmic dysfunctions, and (3) psychosexual impairment with changes in sexual desire, intimacy, and mental health [2]. There has been considerable interest in ED after a prostate cancer diagnosis over the past decade, with an increase in published studies on the subject and an increase in medical coverage [1,2,7,8]. However, treatment outcomes are inconsistent among the treatment options and even among the same treatment option. No consensus exists on the ideal penile rehabilitation regimen, but many urologists agree that treatment should be started as soon as possible to protect or prevent corporal endothelial and smooth muscle damage. The aim of this study was to review the treatment options for ED after RP and to discuss the limitations of each.
Most of the literature indicates that ED after prostate cancer therapy is mainly endemic to the cohort of men who have undergone RP. ED rates af ter RP range from 60% to 70% [2,9,10]. Despite numerous surgical modifications including anatomic nerve-sparing during RP, ED rates in contemporary RP series range from 30% to 87% [1,11,12]. Although anatomic nerve-sparing radical prostatectomy (NSRP) promises a high likelihood of postoperative recovery of ED, many men require more than 2 years to satisfactorily recover erectile function [13].
Recent advances in our knowledge of the functional and topographic anatomy of the prostate and innovations in surgical technology including laparoscopic and robotic surgery have resulted in improved preservation of postoperative erectile function. However, the literature is still insufficient to rigorously compare different surgical techniques and technologies, including the assertion that laparoscopic or robotic RP is better at preventing ED [2,9,10].
The proximity of the cavernosal nerve [14] to the prostatic capsule, which is positioned as a diffuse, poorly visualized nerve plexus adherent with the lateral aspect of the prostate, represents the major surgical obstacle and a limitation of NSRP or advanced surgical devices. Additionally, the small size and dependent location of the cavernous nerve (CN) within the male pelvis make visualization and preservation difficult [15,16]. A review of the literature demonstrates a large discrepancy in ED incidence rates following RP [3,5,17] as a result of intrinsic patient factors, surgical factors, and reporting biases.
Several pathophysiological theories have been proposed to explain ED after RP, including CN injury, vascular compromise such as pudendal artery injury, damage to nearby structures, local inflammatory changes related to surgical effects, cavernosal smooth muscle hypoxia with smooth muscle apoptosis and fibrosis, and corporal venoocclusive dysfunction causing venous leakage [2,10,18].
Well-defined pathophysiological changes are observed in animal models of the penis following CN injury. These pathophysiological changes lead to severe neurapraxia and associated lethal axonal damage, including apoptosis of the smooth muscle and the endothelium of the penis, reduced nitric oxide synthase [19] nerve density, pathobiological signaling responses favoring vasoconstriction, upregulation of fibroproliferative cytokines such as transforming growth factor-beta, and penile smooth muscle fibrosis or loss of smooth muscle [16,20,21,22,23].
The pathophysiology of ED after RP is believed to include neurapraxia, which leads to temporarily reduced oxygenation and subsequent structural changes in penile tissue. This is also related to veno-occlusive dysfunction [3]. Increasing evidence suggests that temporary CN dysfunction leads to structural changes in penile tissue. During the period of neurapraxia, the penile tissue is in a constant state of low oxygen supply, which may lead to smooth muscle apoptosis and fibrosis [24].
Changes in smooth muscle content and increased fibrosis in penile tissue after damage to the CN have been shown in several animal studies. Apoptosis of the smooth muscle is evident as early as 1 day after denervation and is aggravated over time [16,22,25]. These changes are most pronounced in animal models with bilateral CN damage [16,26,27,28,29]. Many animal studies have shown that reduced intracavernosal pressure remains after either injection of vasoactive substances or electrical stimulation [26,27,28,30,31].
The decrease in penile blood flow induces apoptosis of cavernosal and endothelial cells with penile distensibility [16,22,25]. Neurapraxia secondarily causes apoptosis in corpus cavernosal smooth muscle. A change in the ratio of smooth muscle to collagen and the contraction of endotheliocytes could worsen the recovery of erection [26,27,28,30,31].
The presence of transforming growth factor-beta1 [15,21,26] and hypoxia-inducible factor-1α [21], as well as overexpression of endothelin-1 type B receptor [25,32], confirm hypoxia as a possible pathophysiological theory for ED after RP. Oxidative stress has also been noted as a potential contributor [31], whereas a recent study found increased expression of several profibrotic genes and a concomitant decrease in expression of genes promoting smooth muscle growth [33].
To summarize the pathogenesis of ED after RP, neurapraxia induced by unilateral or bilateral CN injury, hypoxia, oxidative stress, and consequent structural changes in the penis are key factors.
Several risk factors are associated with postoperative ED outcomes, including age, the level of erectile function before treatment, the extent of surgical neurovascular preservation, and changes to erectile hemodynamics during surgery [18]. Many studies have shown that higher sexual health-related quality of life scores before treatment, younger age, lower serum prostate-specific antigen levels, race or ethnicity, lower body mass index, and defined intended treatment details are associated with better functional outcomes 2 years after surgery [11].
Penile rehabilitation is defined as "the use of any drug or device after RP to maximize ED recovery" [34]. That is, penile rehabilitation after RP involves the use of any intervention or combination (medications, devices, or actions) to recover erectile function, which help men regain the ability to achieve erections sufficient for satisfactory sexual intercourse during rehabilitation from prostate cancer treatment [3,5]. The rehabilitation treatment strategy should focus on three interrelated concepts: (1) improving cavernosal oxygenation, (2) preserving endothelial structure and function, and (3) preventing smooth muscle structural changes [34,35].
Penile rehabilitation consists of two types of treatment. The first is treating the ED itself and the second is treating penile shape deformities. Most studies have focused on treating ED itself.
Phosphodiesterase type 5 inhibitors (PDE5Is), intracavernosal injections (ICIs) of vasoactive agents (prostaglandins), and vacuum erection devices (VEDs) are options in rehabilitation programs to treat ED. Penile implants should be considered if patients do not respond to medical therapies, and are the only option to treat a shape deformity. To facilitate informed decision-making, patients should be presented with all treatment options and told that rehabilitation and treatment for ED as early as possible after RP will result in faster and better recovery of ED and will preserve sexual continuity [3,5].
The important key point when offering rehabilitation is to initiate treatment as soon as possible after RP, before penile fibrosis develops, which is essential for recovery of erectile function [2,10,36]. The most common strategies for rehabilitation include single agents or a combination of PDE5Is, ICIs, intraurethral injections of vasoactive agents, and VEDs.
First-line treatment includes an oral PDE5I. PDE5Is are quick and easy to administer, discreet, and suitable. However, poor efficacy, particularly in cases with poor CN function, and side effects such as headache, flushing, and palpitations have been reported [2,3].
Second-line treatment options include ICI or intraurethral injection therapy, a VED, or a penile prosthesis, which could be effective for patients who do not respond to oral PDE5I therapy [2]. VEDs are noninvasive but are time-consuming, are expensive, and have many side effects such as bruising, pain, and penile fibrosis [2,3]. ICI or intraurethral injection therapy and a penile prosthesis are invasive procedures [2].
Although recent studies have emphasized the new concept of penile rehabilitation, more effort is needed to demonstrate the optimal indications for this treatment strategy. Namely, it is necessary to identify patient subgroups who do not need and are not suitable for treatment and to identify those who are the best candidates for rehabilitation [3,37,38].
Ideal candidates for penile rehabilitation after RP are patients at intermediate risk for ED [3]. Corpus cavernosal function is already compromised before surgery in patients at high risk for ED; hence, either on-demand or daily PDE5I treatment is ineffective. In contrast, corpus cavernosal function in patients at low risk is sufficient to recover erectile function with an on-demand PDE5I, and daily treatment with a PDE5I may not be required in these patients [37].
Gallina et al. [38] suggested that penile rehabilitation may be beneficial to older patients and patients with diminished preoperative erectile function. They reported that young men (<55 years of age) with good erectile function do not benefit from a rehabilitation strategy. Briganti et al. [37] suggested a new paradigm. They reported that overall recovery of erectile function can be achieved with a PDE5I. In their study, the efficacy of PDE5I was similar between an on-demand and daily method of PDE5I treatment and was similar in patients at high risk (age≥70 years or International Index of Erectile Function-Erectile Function [IIEF-EF] score≤10, or a Charlson Comorbidity Index [39] score≥2) and low risk (age≤65 years, IIEF-EF score≥26, and CCI score≤1) for postoperative ED. Daily treatment showed a significantly better effect in intermediate-risk patients (age 66-69 years; IIEF-EF score of 11-25, and CCI score≤1) [37].
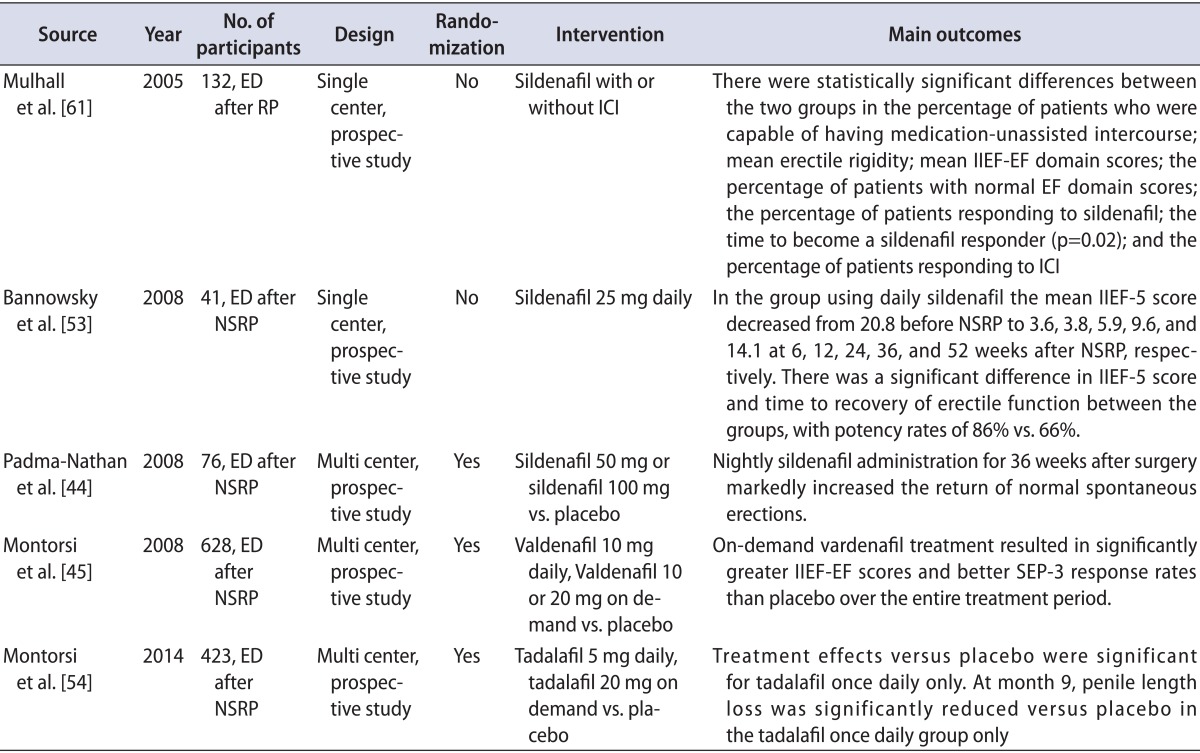
The emergence of PDE5Is has revolutionized ED treatment, and many studies have shown favorable outcomes of PDE5I therapy in patients with ED after NSRP. Various penile rehabilitation programs with a variety of PDE5Is have been used in clinical practice worldwide (Table 1) [40].
No definite evidence is available indicating the best treatment strategy for a penile rehabilitation program using PDE5Is [41], but many urologists agree that PDE5I treatment should commence as soon as possible to prevent the development of structural alterations from prolonged cavernosal hypoxia and subsequent venoocclusive dysfunction from cavernosal fibrosis [5,42]. Although the mechanism of action of PDE5Is depends on preserved cavernosal nerve function [41], one study showed the efficacy of PDE5I treatment even in men who had undergone non-NSRP, thus highlighting the role of nonneuronal stimulation of nitric oxide production on penile erection [43].
Sildenafil affects several genes involved in smooth muscle preservation and in reducing oxidative stress [31,33]. Tadalafil increases activation of the associated kinases [22]. Both expression and activation of endothelial nitric oxide synthase increase with sildenafil treatment compared with that in a control group [28]. Inducible nitric oxide synthase increases with vardenafil treatment [30].
Rare randomized and placebo-controlled trials have been conducted to assess the clinical effects of a PDE5I in patients undergoing penile rehabilitation. In the first trial, men scheduled for bilateral NSRP with intact preoperative erectile function were randomized to receive 100-mg sildenafil, 50-mg sildenafil, or placebo every night for 9 months [44]. Mean IIEF scores were significantly higher in the 50- and 100-mg sildenafil groups than in the placebo group. The limitation of this study was the short follow-up. Enrollment in the study was terminated early because of an interim analysis showing a lower response rate than expected, and only 76 men completed the study protocol.
The second trial was performed by Montorsi et al. [45]. Patients received 10-mg vardenafil nightly plus on-demand placebo, on-demand vardenafil plus nightly placebo, or nightly placebo plus on-demand placebo for 9 months. This study did not demonstrate any significant difference in erectile function among the three groups.
Briganti et al. [37] suggested that differences in preoperative parameters, such as preoperative age, erectile function, and comorbidity profile, which are well-known predictors of postoperative ED [46,47,48,49,50,51,52], may have altered the effect of the PDE5I on erectile function recovery.
Another small randomized study investigated the effect of tadalafil in patients undergoing penile rehabilitation [53]. Sixty-five men with preoperative IIEF-EF scores>25 who were undergoing bilateral NSRP were randomized into two groups to receive 20-mg tadalafil three times per week for 6 months or no treatment. IIEF-EF scores differed between the groups at 52 weeks.
A recent study by Montorsi et al. [54] reported that tadalafil once daily was most effective on drug-assisted EF in men with ED following NSRP. That study also suggested a potential role of tadalafil once daily on the recovery of EF after prostatectomy and possible protection from penile structural changes.
Although there is much positive preclinical study evidence for the effectiveness of PDE5Is in ED after RP (CN injury), only small series have been used in clinical studies. The common limitations in these clinical studies include potential selection bias and lack of biological evidence of erectile function.
A VED is a useful option in men with ED regardless of NSRP or non-NSRP [55]. Moreover, early initiation of daily VED use preserves penile length [56]. However, long-term use of a VED for penile rehabilitation is questionable because of the theoretical risk of aggravating cavernosal fibrosis owing to prolonged ischemia, acidosis, and lack of smooth muscle relaxation [1,5,7].
Although a VED creates a transient increase in arterial blood flow and oxygen supply [7,57,58], oxygen saturation drops gradually when applying a constriction band [57]. Hence, VEDs should be applied without the constriction band for penile rehabilitation.
Two randomized trials have tested the efficacy of VEDs in human trials. Kohler et al. [56] reported that delayed use of a VED does not affect sexual satisfaction once use began. No difference in penile shrinkage was observed once VED use started. Raina et al. [55] reported that the treatment group showed improved sexual satisfaction and a higher rate of spontaneous erections. Several complications have been reported, such as penile discomfort, penile bruising, social inconvenience, and inability to use the device [3].
Use of VEDs lacks evidence in clinical settings.
ICI using alprostadil (a synthetic prostaglandin E1 derivative) either alone or in combination with other agents such as papaverine or phentolamine is a useful second-line treatment option for patients in whom NSRP cannot be achieved or those in whom a PDE5 is ineffective or not tolerated [59]. The first attempt at penile rehabilitation was performed by Montorsi et al. [60]. Twelve patients completed the treatment and eight reported that they needed injection therapy for less than half of their sexual activity attempts, which was considered recovery of spontaneous erection. Injection therapy in patients undergoing penile rehabilitation has not been tested in a randomized fashion since.
The only randomized study investigating intraurethral injection of prostaglandin for penile rehabilitation compared solitary intraurethral injection therapy and combined therapy with nightly sildenafil in 139 patients [60]. No difference in erectile function was observed between the two groups 1 year after RP. Because this study had no placebo or control group, the study cannot be considered adequate documentation of either penile rehabilitation treatment.
Other publications on ICI or intraurethral injection therapies have similar methodological drawbacks. These include comparing patients who actively chose to participate in a penile rehabilitation program with patients who either were not interested or chose to delay treatment and excluding patients who did not comply with treatment regimens [61,62].
A penile implant is a substitutable option for maintaining sexual functioning and preventing subsequent loss of penile length in patients with medically refractive ED [63]. Despite the high satisfaction rate, greater quality of life and erectile function, and higher frequency of sexual contact [64], penile prosthesis implantation has been less of a focus as a secondary treatment option for penile rehabilitation in men with ED related to prostate cancer treatment [65].
Psychological factors such as closeness of relationships and depression or anxiety also have important roles in erectile function after RP [66]. Moreover, a lack of emotional readiness in patients and their partners can negatively affect sexual activity even when erectile function is restored after RP [67]. A strong correlation is observed between male patients with ED after RP and sexual dysfunction of the female partner [68,69].
Canada et al. [70] investigated the effects of four sessions of sexual counseling by men with postoperative ED who had undergone curative treatment for prostate cancer. The counseling program included education on the sexual impact of prostate cancer treatment, information about ED treatments, communication training, and cognitive-behavioral therapy. Patients who completed the program showed short-term improvements in all IIEF subscales except sexual desire. The female partners also showed improvement in sexual function over the short term. However, only 61% completed all four sessions and most of the improvements returned to baseline at the 6-month follow-up in male patients and female partners.
Several treatment options have been introduced but most require additional basic and clinical validation. Among them, testosterone therapy has been a recent focus. Testosterone plays an important role in both CN integrity and nitric oxide production and is thought to support trophic effects on smooth muscle tissue along with reducing fat and connective tissue in the corpora cavernosa [71].
Preoperative serum testosterone levels are positively correlated with ED after RP [72]. Testosterone regulates PDE5I levels in rodents [71] and testosterone may improve the response to PDE5Is in men with hypogonadism [73]. Combined treatment with testosterone and a PDE5I is a promising treatment option for patients undergoing penile rehabilitation. However, the possibility of recurrence or progression of prostate cancer by testosterone must be clarified before widespread use of testosterone in penile rehabilitation.
Neuromodulatory treatments are other viable options. Considerable development in neuromodulatory therapies, such as use of immunophilin ligands, neurotrophins, growth factors, and stem cell therapy, to regenerate the CN and promote axonal regrowth of remaining neural structure has been made over the past decade [5,36]. These neuroregenerative treatments showed promising outcomes in preclinical studies, but basic and clinical studies are needed to clarify long-term efficacy and safety in humans.
Another common complaint among men who have undergone RP is loss of penile length and girth. Contemporary literature reports an approximate loss of 2 to 3 cm of stretched penile length 12 months after RP [74]. This loss of penile length is often accompanied by other penile deformities such as penile curvature [75]. The main mechanism for this early shortening of penile length is parasympathetic neural trauma (cavernosal nerve injury) with subsequent sympathetic neural overdrive and release of various neurotrophic factors [18]. This phenomenon is potentially reversible [3,5]. However, delayed structural changes related to underlying corporal cavernosal smooth muscle hypoxia and denervation-induced smooth muscle apoptosis with fibrosis can worsen penile curvature [2,10].
Many studies lacked essential data such as use of patient self-reported questionnaires, whereas others had too many variables such as definitions for ED and the return of erectile function [1,2,10]. Moreover, studies with biological data such as scans or penile Doppler ultrasonography were scarce.
Although PDE5Is seem efficacious in the CN injury model, clinical studies are insufficient to provide further evidence. Moreover, only two randomized controlled studies have been conducted with possibly high selection bias. More investigations are crucial to determine long-term efficacy and safety. No consensus exists on the appropriate PDE5I, dose, and regimen, but PDE5Is have a role in combined therapy and are indicated for some patients. More basic studies and clinical studies are needed for second-line treatments.
No standard treatment strategy is available for ED penile rehabilitation after RP. No attempts have been made to investigate the efficacy of a systematic treatment strategy for penile rehabilitation. Much effort has been made toward establishing a standard treatment strategy, particularly considering psychological and social impacts.
Clinicians must discuss the pros and cons of penile rehabilitation with patients undergoing RP. Patients should be informed about the negative consequences of long-term ED after RP [35].
Although the role of penile rehabilitation using PDE5Is as a first-line treatment option continues to evolve, current clinical trials have significant limitations. Most urologists agree that oral PDE5I therapy should be started as soon as possible to protect against or prevent corporal endothelial and smooth muscle damage. Second-line therapies such as ICI or intraurethral injection of vasoactive agents and VEDs should be offered to patients who did not undergo NSRP and who wish to remain sexually active. ICI or intraurethral injection of vasoactive agents and VEDs are also viable options for patients who underwent NSRP and did not show satisfactory results with oral PDE5Is. A penile implant should be considered to preserve penile length when patients fail to respond to medical therapies. Although each treatment option has limitations, penile rehabilitation may have potential benefits for the patient and his partner and should be considered after RP.
References
1. Tal R, Alphs HH, Krebs P, Nelson CJ, Mulhall JP. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009; 6:2538–2546. PMID: 19515209.

2. Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med. 2013; 10(Suppl 1):102–111. PMID: 23387915.

3. Fode M, Ohl DA, Ralph D, Sonksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013; 112:998–1008. PMID: 23826962.

4. Chen RC, Clark JA, Manola J, Talcott JA. Treatment 'mismatch' in early prostate cancer: do treatment choices take patient quality of life into account? Cancer. 2008; 112:61–68. PMID: 18040996.
5. Chung E, Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med J Aust. 2014; 200:582–585. PMID: 24882489.

6. Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012; 62:418–430. PMID: 22749850.

7. Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol. 2012; 62:273–286. PMID: 22575910.

8. Salonia A, Gallina A, Zanni G, Briganti A, Deho F, Sacca A, et al. Acceptance of and discontinuation rate from erectile dysfunction oral treatment in patients following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008; 53:564–570. PMID: 17761385.

9. Mulhall JP, Morgentaler A. Penile rehabilitation should become the norm for radical prostatectomy patients. J Sex Med. 2007; 4:538–543. PMID: 17498093.
10. Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol. 2012; 62:261–272. PMID: 22575909.

11. Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, Sandler HM, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011; 306:1205–1214. PMID: 21934053.

12. Burnett AL. Erectile dysfunction following radical prostatectomy. JAMA. 2005; 293:2648–2653. PMID: 15928287.

13. Rabbani F, Schiff J, Piecuch M, Yunis LH, Eastham JA, Scardino PT, et al. Time course of recovery of erectile function after radical retropubic prostatectomy: does anyone recover after 2 years? J Sex Med. 2010; 7:3984–3990. PMID: 20722784.

14. Bivalacqua TJ, Usta MF, Champion HC, Leungwattanakij S, Dabisch PA, McNamara DB, et al. Effect of combination endothelial nitric oxide synthase gene therapy and sildenafil on erectile function in diabetic rats. Int J Impot Res. 2004; 16:21–29. PMID: 14963467.

15. Hu WL, Hu LQ, Song J, Li SW, Zheng XM, Cheng B, et al. Fibrosis of corpus cavernosum in animals following cavernous nerve ablation. Asian J Androl. 2004; 6:111–116. PMID: 15154084.
16. User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003; 169:1175–1179. PMID: 12576876.

17. Kaiho Y, Yamashita S, Arai Y. Optimization of sexual function outcome after radical prostatectomy using phosphodiesterase type 5 inhibitors. Int J Urol. 2013; 20:285–289. PMID: 23311962.

18. Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010; 7(4 Pt 2):1687–1698. PMID: 20388165.

19. Nossaman BD, Gur S, Kadowitz PJ. Gene and stem cell therapy in the treatment of erectile dysfunction and pulmonary hypertension; potential treatments for the common problem of endothelial dysfunction. Curr Gene Ther. 2007; 7:131–153. PMID: 17430132.

20. Gratzke C, Strong TD, Gebska MA, Champion HC, Stief CG, Burnett AL, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J Urol. 2010; 184:2197–2204. PMID: 20851436.

21. Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, Champion HC, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003; 24:239–245. PMID: 12634311.

22. Lysiak JJ, Yang SK, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008; 179:779–785. PMID: 18082193.

23. Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006; 3:77–83. PMID: 16409220.

24. Moreland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res. 1998; 10:113–120. PMID: 9647948.

25. Vignozzi L, Morelli A, Filippi S, Vannelli GB, Mungai S, Marini M, et al. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int J Impot Res. 2008; 20:60–67. PMID: 17703219.

26. Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, Rajfer J, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008; 20:202–212. PMID: 17882231.

27. Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, Rajfer J, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008; 101:203–210. PMID: 17888043.

28. Mulhall JP, Muller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008; 5:1126–1136. PMID: 18331274.

29. Ozden E, Ozturk B, Kosan M, Tezel GG, Aki FT, Gur S, et al. Effect of sildenafil citrate on penile weight and physiology of cavernous smooth muscle in a post-radical prostatectomy model of erectile dysfunction in rats. Urology. 2011; 77:761.e1–761.e7. PMID: 21256544.

30. Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006; 68:429–435. PMID: 16904479.

31. Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007; 4(4 Pt 1):908–916. PMID: 17627738.

32. Vignozzi L, Filippi S, Morelli A, Ambrosini S, Luconi M, Vannelli GB, et al. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med. 2006; 3:419–431. PMID: 16681467.
33. Sirad F, Hlaing S, Kovanecz I, Artaza JN, Garcia LA, Rajfer J, et al. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med. 2011; 8:1048–1060. PMID: 21269401.

34. Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008; 18:613–620. PMID: 18832948.

35. Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013; 10:195–203. PMID: 22970890.

36. Chung E, Brock GB. Emerging and novel therapeutic approaches in the treatment of male erectile dysfunction. Curr Urol Rep. 2011; 12:432–443. PMID: 21922167.

37. Briganti A, Di Trapani E, Abdollah F, Gallina A, Suardi N, Capitanio U, et al. Choosing the best candidates for penile rehabilitation after bilateral nerve-sparing radical prostatectomy. J Sex Med. 2012; 9:608–617. PMID: 22189164.

38. Gallina A, Ferrari M, Suardi N, Capitanio U, Abdollah F, Tutolo M, et al. Erectile function outcome after bilateral nerve sparing radical prostatectomy: which patients may be left untreated? J Sex Med. 2012; 9:903–908. PMID: 22240189.

39. Gacci M, Bartoletti R, Figlioli S, Sarti E, Eisner B, Boddi V, et al. Urinary symptoms, quality of life and sexual function in patients with benign prostatic hypertrophy before and after prostatectomy: a prospective study. BJU Int. 2003; 91:196–200. PMID: 12581003.

40. Teloken P, Mesquita G, Montorsi F, Mulhall J. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the international society for sexual medicine practitioners. J Sex Med. 2009; 6:2032–2038. PMID: 19453918.
41. Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009; 55:334–347. PMID: 18986755.

42. Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009; 6(Suppl 3):353–362. PMID: 19267860.
43. Garcia-Cardoso J, Vela R, Mahillo E, Mateos-Caceres PJ, Modrego J, Macaya C, et al. Increased cyclic guanosine monophosphate production and endothelial nitric oxide synthase level in mononuclear cells from sildenafil citrate-treated patients with erectile dysfunction. Int J Impot Res. 2010; 22:68–76. PMID: 19907424.

44. Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008; 20:479–486. PMID: 18650827.

45. Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008; 54:924–931. PMID: 18640769.

46. Ayyathurai R, Manoharan M, Nieder AM, Kava B, Soloway MS. Factors affecting erectile function after radical retropubic prostatectomy: results from 1620 consecutive patients. BJU Int. 2008; 101:833–836. PMID: 18190627.

47. Briganti A, Capitanio U, Chun FK, Karakiewicz PI, Salonia A, Bianchi M, et al. Prediction of sexual function after radical prostatectomy. Cancer. 2009; 115(13 Suppl):3150–3159. PMID: 19544544.

48. Descazeaud A, Debre B, Flam TA. Age difference between patient and partner is a predictive factor of potency rate following radical prostatectomy. J Urol. 2006; 176(6 Pt 1):2594–2598. PMID: 17085166.

49. Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008; 179:2207–2210. PMID: 18423693.

50. Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004; 172(6 Pt 1):2227–2231. PMID: 15538237.

51. Michl UH, Friedrich MG, Graefen M, Haese A, Heinzer H, Huland H. Prediction of postoperative sexual function after nerve sparing radical retropubic prostatectomy. J Urol. 2006; 176:227–231. PMID: 16753406.

52. Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000; 164:1929–1934. PMID: 11061884.

53. Bannowsky A, Schulze H, van der Horst C, Hautmann S, Junemann KP. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. BJU Int. 2008; 101:1279–1283. PMID: 18284406.

54. Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, Patel HR, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). Eur Urol. 2014; 65:587–596. PMID: 24169081.

55. Raina R, Agarwal A, Ausmundson S, Lakin M, Nandipati KC, Montague DK, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006; 18:77–81. PMID: 16107868.

56. Kohler TS, Pedro R, Hendlin K, Utz W, Ugarte R, Reddy P, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007; 100:858–862. PMID: 17822466.

57. Bosshardt RJ, Farwerk R, Sikora R, Sohn M, Jakse G. Objective measurement of the effectiveness, therapeutic success and dynamic mechanisms of the vacuum device. Br J Urol. 1995; 75:786–791. PMID: 7613837.

58. Broderick GA, McGahan JP, Stone AR, White RD. The hemodynamics of vacuum constriction erections: assessment by color Doppler ultrasound. J Urol. 1992; 147:57–61. PMID: 1729552.

59. Raina R, Lakin MM, Thukral M, Agarwal A, Ausmundson S, Montague DK, et al. Long-term efficacy and compliance of intracorporeal (IC) injection for erectile dysfunction following radical prostatectomy: SHIM (IIEF-5) analysis. Int J Impot Res. 2003; 15:318–322. PMID: 14562131.

60. Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997; 158:1408–1410. PMID: 9302132.

61. Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005; 2:532–540. PMID: 16422848.

62. Mulhall JP, Parker M, Waters BW, Flanigan R. The timing of penile rehabilitation after bilateral nerve-sparing radical prostatectomy affects the recovery of erectile function. BJU Int. 2010; 105:37–41. PMID: 19659465.

63. Stephenson RA, Mori M, Hsieh YC, Beer TM, Stanford JL, Gilliland FD, et al. Treatment of erectile dysfunction following therapy for clinically localized prostate cancer: patient reported use and outcomes from the Surveillance, Epidemiology, and End Results Prostate Cancer Outcomes Study. J Urol. 2005; 174:646–650. PMID: 16006930.

64. Ramsawh HJ, Morgentaler A, Covino N, Barlow DH, DeWolf WC. Quality of life following simultaneous placement of penile prosthesis with radical prostatectomy. J Urol. 2005; 174(4 Pt 1):1395–1398. PMID: 16145445.

65. Tal R, Jacks LM, Elkin E, Mulhall JP. Penile implant utilization following treatment for prostate cancer: analysis of the SEER-Medicare database. J Sex Med. 2011; 8:1797–1804. PMID: 21426495.

66. Nelson CJ, Choi JM, Mulhall JP, Roth AJ. Determinants of sexual satisfaction in men with prostate cancer. J Sex Med. 2007; 4:1422–1427. PMID: 17634054.
67. Son H, Park K, Kim SW, Paick JS. Reasons for discontinuation of sildenafil citrate after successful restoration of erectile function. Asian J Androl. 2004; 6:117–120. PMID: 15154085.
68. Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002; 95:1773–1785. PMID: 12365027.

69. Shindel A, Quayle S, Yan Y, Husain A, Naughton C. Sexual dysfunction in female partners of men who have undergone radical prostatectomy correlates with sexual dysfunction of the male partner. J Sex Med. 2005; 2:833–841. PMID: 16422807.

70. Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005; 104:2689–2700. PMID: 16294343.

71. Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006; 3:382–404. PMID: 16681465.
72. Kim SC, Song C, Kim W, Kang T, Park J, Jeong IG, et al. Factors determining functional outcomes after radical prostatectomy: robot-assisted versus retropubic. Eur Urol. 2011; 60:413–419. PMID: 21612859.

73. Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2008; 179(5 Suppl):S97–S102. PMID: 18405769.

74. McCullough A. Penile change following radical prostatectomy: size, smooth muscle atrophy, and curve. Curr Urol Rep. 2008; 9:492–499. PMID: 18947515.

75. Tal R, Heck M, Teloken P, Siegrist T, Nelson CJ, Mulhall JP. Peyronie's disease following radical prostatectomy: incidence and predictors. J Sex Med. 2010; 7:1254–1261. PMID: 20500447.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download