Abstract
Purpose
Many patients admitted for acute myocardial infarction (AMI) have chronic renal insufficiency and erectile dysfunction (ED). This study aimed to evaluate the relationship between ED and the glomerular filtration rate (GFR) in patients with coronary artery disease.
Materials and Methods
We studied 183 patients undergoing coronary angiography owing to AMI. The GFR was calculated and the International Index of Erectile Function-5 (IIEF-5) was used to evaluate ED. The relations between erectile function, GFR, and the number of occluded coronary arteries were evaluated.
Results
Of 183 patients with a mean age of 55.2±11.16 years who underwent coronary angiography owing to AMI, 100 (54.64%) had ED. The ED rate was 45.36% (44/97) in patients with single-vessel disease, 64.5% (31/48) in patients with two-vessel disease, and 65.7% (25/38) in patients with three-vessel disease. The ED rate in patients with single-vessel disease was significantly lower than in the other groups (p<0.001). The mean IIEF scores were 24.2±4.3, 20.4±4.9, and 20.5±4.2 in the three groups, respectively (p<0.001). Mean GFRs were similar in patients with single-vessel disease, two-vessel disease, and three-vessel disease (128.2±46.8, 130.8±70.9, and 110.8±44.6, respectively, p=0.171). The GFR was significantly lower in the presence of ED only for single-vessel disease (p=0.001).
Conclusions
This study confirmed that the presence and severity of ED are linked to the number of occluded vessels as documented by coronary angiography. The presence of ED and reduced GFR are associated with single-vessel coronary artery disease. This relationship can be used to predict the likelihood of coronary artery disease.
Erectile dysfunction (ED) is defined as the consistent inability to obtain and maintain an erection satisfactory for sexual activity [1]. ED and cardiovascular diseases share common risk factors such as cigarette smoking, obesity, metabolic syndrome, and a sedentary way of life. Also, we know that coronary artery disease (CAD) is the leading cause of death in the Western world, causing serious morbidity [2]. Epidemiological surveys have highlighted the relationship between cardiovascular disease risk factors and sexual dysfunction in men [3]. ED occurs after various pathophysiological mechanisms and has been associated with a high component of endothelial dysfunction, such as CAD, idiopathic systemic arterial hypertension, and chronic kidney disease (CKD) [4]. Moreover, endothelial dysfunction is an early marker of CAD and has also been reported to occur in CKD patients [5]. Recently, CKD has also gained attention as a risk factor for ED. After all is said and done, ED and CKD are recognized as potential independent risk factors or predictors of CAD. The aim of this study was to evaluate the association between the glomerular filtration rate (GFR), the severity of ED, and the severity of CAD in patients with a diagnosis of myocardial infarction (MI) by coronary angiography.
A total of 183 consecutive men who underwent coronary angiography because of acute MI from November 2009 to May 2011 were included in the study. The study protocol was designed in adherence to the Declaration of Helsinki and written informed consent was obtained from each patient. The local ethics committee approved the study. During the angiography, CAD was defined as an occlusion greater than 50% in the main coronary artery or an occlusion greater than 70% in any of the other coronary arteries, and the number of occluded arteries was noted [6].
All patients who stabilized after MI were evaluated by the erectile function domain of the International Index of Erectile Function (IIEF), a validated 15-item selfadministered questionnaire that was validated in Turkish [7]. An IIEF-5 score below 22 was considered as ED [8]. The GFR was calculated by using the Cockcroft-Gault equation [9].
In order to determine both cardiovascular and ED risk factors, all male patients who underwent angiography were evaluated with regard to parameters such as age, cigarette smoking (patients who smoked more than 10 cigarettes a day were considered smokers, and patients who ceased smoking more than 2 years ago were considered nonsmokers), waist circumference, levels of total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride. Hypertension and diabetes mellitus were evaluated according to the Seventh Report of the Joint National Committee [10].
The drugs used by the patients (beta-blockers, angiotensin-converting enzyme [ACE] inhibitors, calcium channel blockers, diuretics, nitrates, insulin, oral antidiabetic agents, statins, and acetyl salicylic acid) were noted. Patients with other chronic drug use were not included in the study. The other exclusion criteria were as follows: previous MI; coronary artery bypass grafting surgery or percutaneous coronary angioplasty; renal replacement therapy; known endocrinologic, neurologic, or psychiatric disease; and history of pelvic-urethral-prostatic surgery. Furthermore, patients who were undergoing a treatment because of ED were not included in our study.
Statistical analyses were performed by using the Statistical Package for the IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). The analyses were performed by use of the Kolmogorov-Smirnov test, analysis of variance and t-test, Kruskal-Wallis test, Mann-Whitney U test, chi-square test, and Fischer test. Categorical data were studied by Pearson correlation analysis, whereas discrete data were evaluated by Spearman correlation analysis. In all statistical analyses, a p-value less than 0.05 was accepted to be statistically significant.
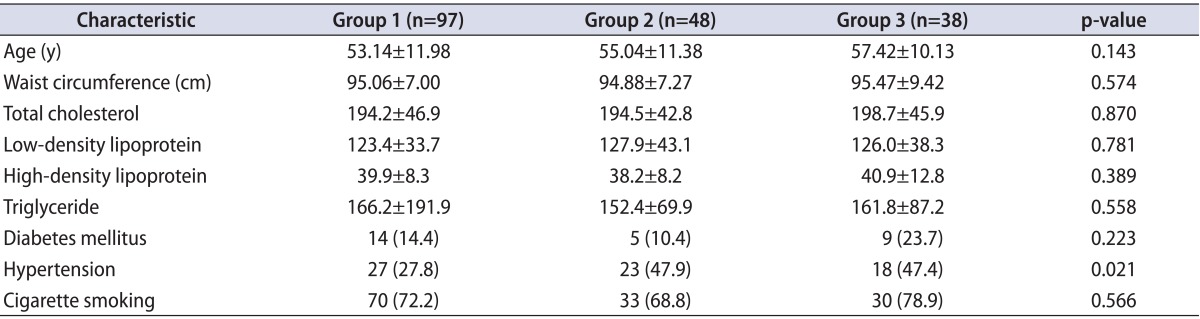
The 183 patients who underwent coronary angiography were categorized in three groups: single-vessel occlusion (group 1), two-vessel occlusion (group 2), and three-vessel occlusion (group 3). Group 1 had 97 patients, group 2 had 48 patients, and group 3 had 38 patients. The demographic characteristics of the groups are shown in Table 1.
There were no statistically significant differences between the groups with respect to age, waist circumference, serum cholesterol and triglyceride concentrations, smoking status, or the number of patients with diabetes. Only the rate of hypertension was significantly higher in the two-vessel and three-vessel occlusion groups than in the single-vessel occlusion group.
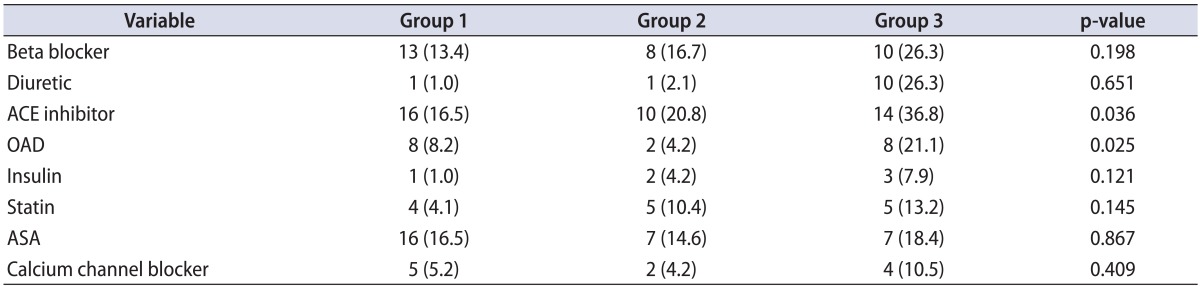
The use of drugs that could affect erectile function is shown in detail in Table 2. Only the use of ACE inhibitors and oral antidiabetics was significantly higher in group 3.
Of the 183 patients diagnosed with AMI, 100 (54.64%) had various degrees of ED. Rates of ED were 45.36% (44/97) for group 1, 64.5% (31/48) for group 2, and 65.7% (25/38) for group 3. The ED rate in group 1 was significantly lower than that in groups 2 and 3 (p<0.001). The mean IIEF-5 scores were 24.2±4.3 in group 1, 20.4±4.9 in group 2, and 20.5±4.2 in group 3 (p<0.001). There was no significant difference between groups 2 and 3 with regard to IIEF-5 scores (Fig. 1).
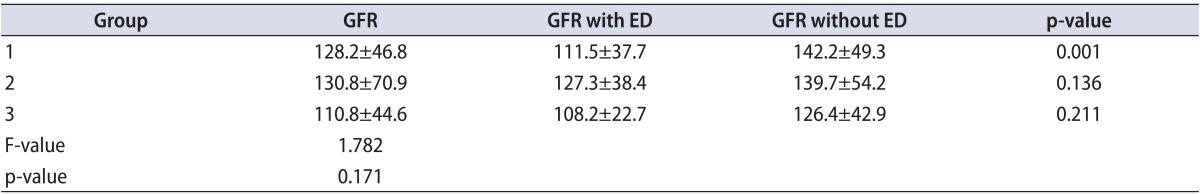
When renal function was examined, mean GFRs were similar in patients with single-vessel disease, two-vessel disease, and three-vessel disease (128.2±46.8, 130.8±70.9, and 110.8±44.6, respectively, p=0.171). The GFRs of the groups in the presence and absence of ED are shown in Table 3. The GFR was significantly lower in the presence of ED for single-vessel disease only. The GFR was not associated with the presence or absence of ED in patients with twoor three-vessel disease.
Diffuse atherosclerotic processes cause major pathophysiologic changes in cardiovascular and peripheral arterial diseases. ED has been associated with a high component of endothelial dysfunction, such as CAD and CKD. In one study, most of the patients who had CAD also had ED, and in 70% of these patients, ED was present before anginal symptoms became evident [11]. In our study, 54.64% of 183 patients diagnosed with AMI had various degrees of ED. The presence of ED can be an alternative marker or an independent risk factor for CAD. Another study showed a significant correlation between ischemic heart disease and ED; of 417 patients with ED, 27.6% had mild, 30% had moderate, and 42.4% had severe ED. In that study, 26.9% of the patients were found to have various degrees of ischemic heart disease, and the severity of ED was observed to be significantly elevated in patients with ischemic heart disease of high degree [12]. Our study showed that erectile function in patients with two- and three-vessel disease was worse than that in patients with single-vessel disease by coronary angiography. In this way, the examination of patients with other risk factors for the presence or severity of ED may give some clues to the presence of prior CAD. Moreover, Billups at al. [13] demonstrated that the small diameter of the cavernosal arteries and the high content of endothelium per gram of tissue may make the penile vascular bed a sensitive and early indicator of systemic vascular disease.
CKD is also associated with inflammation and the subsequent endothelial dysfunction [14]. Many studies have shown a relationship between CAD and CKD [15,16]. Nakamura at al. [17] reported that cardiovascular events, including cardiac death and nonfatal MI, increase significantly with declining estimated GFR.
The prevalence of ED in patients with chronic renal failure is also known. Makarem at al. [18] demonstrated that the prevalence of ED in men with CKD under hemodialysis was 86.6%. In addition to the progression of atherosclerosis in chronic renal failure, hyperprolactinemia, lowered serum testosterone levels, drugs, increased parathyroid hormone serum levels, zinc deficiency, and psychological factors were associated with the development of ED in these patients. However, the prevalence of ED is increased not only in patients with CKD. Kopp et al. [19] concluded that patients undergoing radical nephrectomy had significantly higher de novo ED than did patients undergoing partial nephrectomy.
Some studies have shown that ED and CAD are prevalent among patients with CKD [20,21]. Only a few studies in the literature have taken into consideration the relationship between ED and CKD in patients with CAD. In one study, patients presenting with chest pain underwent an exercise stress test (EST), and EST (+) patients underwent coronary angiography and recording of their GFR values. The study showed that the presence and severity of ED are related to the severity of CAD and that lower creatinine clearance is associated with more severe CAD [22]. However, in that study, some patients with significant coronary lesions may have been missed because of negative results on the EST. All patients included in our study were shown to have critically stenotic atheromatous plaques after coronary angiography. The rate of patients with ED was significantly lower among patients with single-vessel disease than among patients with two- or three-vessel disease. Also, the GFR was lower in ED patients only for single-vessel disease. The GFR was not associated with ED in patients with two- or three-vessel disease. This may be explained by the relatively small number of patients.
Vascular disease and ED have a similar pathogenic correlation with the nitric oxide pathway, leading to disruption of endothelium-dependent structural vascular abnormalities [23,24]. An animal modeling study showed that impairment of erection in chronic renal failure in the rat is attributable to a disturbance in nitric oxide synthase gene expression [25]. Thus, perhaps in the future, phosphodiesterase type 5 inhibitors will have potential clinical value in the treatment of CKD with CAD and ED.
The GFR is currently considered the best overall index of renal function. Validation studies have shown that the Cockcroft-Gault formula is equivalent to or superior than various other measurements of GFR (e.g., clearances of insulin, iothalamate, Cr51-ethylenediaminetetraacetic acid, diethylenetriaminepentaacetic acid, Mag3, iohexol) [26]. Therefore, we used the GFR to evaluate renal function and calculated it by use of this formula.
A low serum testosterone level is known to be associated with the severity of ED and CKD. The most important limitation of this study may be the lack of considered levels of testosterone. Drugs that might affect erectile function were also shown in our study. The drug usage of each of the groups was similar, except for ACE inhibitors and oral antidiabetics. The GFR can be affected by these two groups of drugs, which may be another limitation of our study.
Erectile function after acute MI was significantly more deficient in patients with two- and three-vessel disease than in patients with single-vessel disease. We also showed that the GFR was significantly lower in the presence of ED in patients with single-vessel disease. In our opinion, the presence and severity of ED and a low GFR can be used together to predict the likelihood of CAD. However, large-scale and randomized studies are necessary to determine the relationship between the severity of CAD and the severity of ED and the GFR.
References
2. Harriss LR, Ajani AE, Hunt D, Shaw J, Chambers B, Dewey H, et al. Accuracy of national mortality codes in identifying adjudicated cardiovascular deaths. Aust N Z J Public Health. 2011; 35:466–476. PMID: 21973254.

3. Laumann EO, Paik A, Rosen RC. The epidemiology of erectile dysfunction: results from the National Health and Social Life Survey. Int J Impot Res. 1999; 11(Suppl 1):S60–S64. PMID: 10554933.
4. Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Tsekoura D, Vasiliadou C, et al. Arterial function and intimamedia thickness in hypertensive patients with erectile dysfunction. J Hypertens. 2008; 26:1829–1836. PMID: 18698219.

5. Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011; 26:3537–3543. PMID: 21378154.

6. Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994; 330:1782–1788. PMID: 8190154.

7. Turunc T, Deveci S, Guvel S, Peskircioglu L. The assessment of Turkish validation with 5 question version of ınternation index of erectile function (IIEF-5). Turkish J Urol. 2007; 33:45–49.
8. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999; 11:319–326. PMID: 10637462.

9. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31–41. PMID: 1244564.

10. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42:1206–1252. PMID: 14656957.

11. Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003; 44:360–364. PMID: 12932937.

12. El-Sakka AI, Morsy AM, Fagih BI, Nassar AH. Coronary artery risk factors in patients with erectile dysfunction. J Urol. 2004; 172:251–254. PMID: 15201787.

13. Billups KL, Bank AJ, Padma-Nathan H, Katz S, Williams R. Erectile dysfunction is a marker for cardiovascular disease: results of the minority health institute expert advisory panel. J Sex Med. 2005; 2:40–50. PMID: 16422903.
14. Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003; 41:1212–1218. PMID: 12776273.

15. El Barzouhi A, Elias-Smale S, Dehghan A, Vliegenthart-Proenca R, Oudkerk M, Hofman A, et al. Renal function is related to severity of coronary artery calcification in elderly persons: the Rotterdam study. PLoS One. 2011; 6:e16738. PMID: 21311747.

16. Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010; 55:21–30. PMID: 19765871.

17. Nakamura M, Yamashita T, Yajima J, Oikawa Y, Ogasawara K, Kirigaya H, et al. Impact of reduced renal function on prognosis in Japanese patients with coronary artery disease: a prospective cohort of Shinken Database 2007. Hypertens Res. 2009; 32:920–926. PMID: 19696780.

18. Makarem AR, Karami MY, Zekavat OR. Erectile dysfunction among hemodialysis patients. Int Urol Nephrol. 2011; 43:117–123. PMID: 20535636.

19. Kopp RP, Dicks BM, Goldstein I, Mehrazin R, Silberstein JL, Colangelo CJ, et al. Does radical nephrectomy increase the risk of erectile dysfunction compared with partial nephrectomy? A cohort analysis. BJU Int. 2013; 111(3 Pt B):E98–E102. PMID: 22757628.

20. Bellinghieri G, Santoro D, Mallamace A, Savica V. Sexual dysfunction in chronic renal failure. J Nephrol. 2008; 21(Suppl 13):S113–S117. PMID: 18446743.
21. Messina LE, Claro JA, Nardozza A, Andrade E, Ortiz V, Srougi M. Erectile dysfunction in patients with chronic renal failure. Int Braz J Urol. 2007; 33:673–678. PMID: 17980064.
22. Solak Y, Akilli H, Atalay H, Kayrak M, Gok H, Turk S. The association of glomerular filtration rate and erectile dysfunction with severity of coronary artery disease in patients presenting with chest pain. Int Urol Nephrol. 2010; 42:765–771. PMID: 20039124.

23. Azadzoi KM, Goldstein I. Erectile dysfunction due to atherosclerotic vascular disease: the development of an animal model. J Urol. 1992; 147:1675–1681. PMID: 1593719.

24. Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003; 89:251–253. PMID: 12591819.

25. Abdel-Gawad M, Huynh H, Brock GB. Experimental chronic renal failure-associated erectile dysfunction: molecular alterations in nitric oxide synthase pathway and IGF-I system. Mol Urol. 1999; 3:117–125. PMID: 10851313.
26. Johnson D. Caring for Australians with Renal Impairment (CARI). The CARI guidelines: evaluation of renal function. Nephrology (Carlton). 2005; 10(Suppl 4):S133–S176. PMID: 16221118.
Fig. 1
Relationship between International Index of Erectile Function-5 (IIEF-5) scores and number of occluded vessels. Group 1, single-vessel occlusion; group 2, two-vessel occlusion; group 3, three-vessel occlusion.
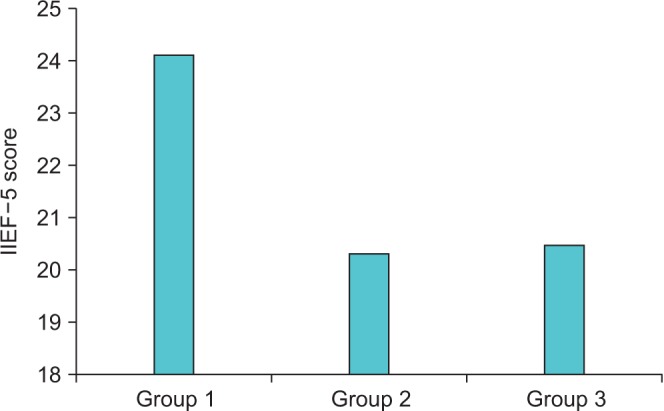




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download