Abstract
Local recurrence after radical nephroureterectomy (RNU) owing to urothelial carcinoma of the upper urinary tract is rare. The usual treatment is systemic chemotherapy followed by optional resection of the mass. We introduce the case of a 73-year-old male patient with multiple comorbidities in whom retroperitoneal carcinoma recurrence of 31 mm was diagnosed via positron emission tomography-computed tomography scan with 18-fluorodeoxyglucose about 5 years after he had undergone RNU owing to urothelial carcinoma of the upper urinary tract. The patient was treated with computed tomography-guided percutaneous radiofrequency ablation. Later scans with contrast controls showed lack of contrast uptake and a decrease of the lesion's size. Twenty-four months after the procedure, the patient is free of the disease. To date, this is the first case of recurrence of urothelial carcinoma that was treated with percutaneous radiofrequency ablation, thus establishing an alternative to chemotherapy in patients with substantial comorbidities.
Urothelial carcinomas of the upper urinary tract make up approximately 10% of kidney tumors. Nowadays, the preferred treatment is either open or laparoscopic radical nephroureterectomy (RNU) with excision of a perimeatal bladder cuff, without any significant difference in terms of oncological efficiency between these approaches [1].
Muscle-invasive urothelial carcinomas show an aggressive behavior and tend to urothelial, retroperitoneal, and distant recurrence with a 5-year global cancer-specific survival of less than 50% [1]. Isolated retroperitoneal recurrence of nephroureterectomy-treated urothelial carcinoma is infrequent, making up 9% of recurrences. Traditionally, the treatment of these recurrences consists of the administration of platinum-based systemic chemotherapy, with cancer-specific survival of 8 to 20 months [2].
Here we introduce the first case to our knowledge of retroperitoneal recurrence after nephroureterectomy that was treated with percutaneous radiofrequency ablation.
A 73-year-old male patient with a medical history of hypertension, diabetes mellitus, ischemic cardiopathology of acute myocardial infarction, and chronic renal failure with a glomerular filtration rate of less than 30 had been treated 5 years previously with right laparoscopic RNU with open ureteral disinsertion owing to a pelvic tumor diagnosed by computed tomography (CT). During the RNU procedure there were neither intraoperative complications nor any openings in the urinary tract. The specimen was removed en bloc alongside a perimeatal bladder cuff. The anatomopathological study of the specimen revealed transitional cell carcinoma pT2G3N0M0. Subsequent examinations were carried out via CT scans, urine cytology, and cystoscopy.
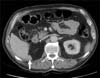
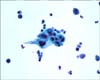
Three years later, during a follow-up CT scan, the patient showed a 31-mm long lesion with contrast uptake in his right renal bed contiguous to the medial border of the right hepatic lobe (Fig. 1). A positron emission tomography (PET)-CT scan with 18-fluorodeoxyglucose was performed, revealing metabolic activity in the surgical bed. After this discovery, a percutaneous biopsy was performed. The anatomopathological diagnosis was high-grade urothelial carcinoma, and the patient was diagnosed with retroperitoneal recurrence of urothelial carcinoma (Fig. 2).
Given the patient's comorbidities, systemic chemotherapy treatment was dismissed in favor of performing radiofrequency ablation on the lesion. The procedure was performed via a CT-guided paravertebral percutaneous puncture under intravenous sedation with the patient in the prone position. The ablation was executed with a 250-W radiofrequency generator and a 3-cm semiflexible electrode. There were no complications during the procedure, and the patient was discharged the day after surgery.
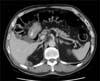
Two years after the procedure, PET-CT and CT scans with contrast controls showed a 10-mm decrease in the original size of the lesion with a lack of contrast uptake (Fig. 3). The results of a percutaneous biopsy of the residual mass showed necrosis without evidence of malignancy. The patient is asymptomatic without tumor recurrence in any other location.
Local recurrence of urothelial carcinoma after RNU is a rare phenomenon with a variable incidence according to the series. In a review of 18 case studies, Rassweiler et al. [3] found incidence rates of local recurrence after open and laparoscopic RNU ranging from 0% to 15%, whereas Hattori et al. [4] described incidence rates of 10% and 6% for open and laparoscopic RNU, respectively, without any statistically significant difference between the two methods. The factors that increase the risk of local recurrence in laparoscopic surgery are the opening of the urinary tract, direct contact to the tumor with instruments, and morcellation of the surgical specimen without bag extraction [3].
We can find multiple studies within the medical literature that analyze the factors predictive of recurrence after radical surgery with curative intent. The most commonly accepted of these factors are local stage, degree of lymph node alteration, cell grade, presence of lymphovascular invasion, and previous history of bladder tumor. Other factors such as the ureteral location of the tumor or the strictly laparoscopic handling of the distal ureter have only been acknowledged as predictive factors by certain authors [5].
The presence of retroperitoneal recurrence of urothelial carcinoma has traditionally been linked to an adverse oncological prognosis. Only two specific studies have been reported on the treatment of recurrence of upper urinary tract tumors. In Hall et al. [2]'s research, six patients with local recurrence after nephroureterectomy were treated with systemic chemotherapy exclusively, with all patients dying before 37 months. In Childs et al. [6]'s study, four patients with retroperitoneal lymph node recurrence were treated with systemic chemotherapy first and then with salvage bilateral retroperitoneal lymphadenectomy. Two of the patients are still alive and free of the disease 56 and 74 months after the procedure, respectively, whereas the other two passed away as a result of the disease course 3 and 42 months after the procedure, respectively.
Other series have analyzed the usefulness of surgery for treating metastases from urothelial tumors, including bladder and upper urinary tract tumors, lymph node metastasis, and solid organ metastasis. In a study with 11 patients about the usefulness of surgery in lymph node metastases of urothelial carcinoma after major response to chemotherapy, Sweeney et al. [7] reported survival of 57, 58, and 8 months in patients in whom the primary tumor was located in the upper urothelium. Abe et al. [8] analyzed survival in 48 patients with metastatic urothelial carcinoma undergoing metastasectomy after response to neoadjuvant chemotherapy. In that study, 24 cases were upper urinary tract tumors, of which only 8 showed sufficient response to chemotherapy for metastasectomy, reaching survival times of 18 to 92 months with a median of 42 months. The group of Lehmann et al. [9] included 44 patients from 15 oncologic German centers with metastatic urothelial cancer. Nine of these patients had upper urothelial tumors, and 80% percent of the cases had undergone systemic chemotherapy before or after metastases resection. In this group, the median survival was 34 months and the 5-year survival was 28% for all tumors.
CT-guided percutaneous radiofrequency ablation has become a viable option for the treatment of kidney tumors less than 4 cm in patients with significant comorbidities or renal failure or in those who refuse surgery, with 5-year disease-free survival rates of 83% [10]. We have not found any publications in which this technique was used in the treatment of primary urothelial carcinoma or in the treatment of recurrences after surgical treatment.
In our case, surgery was dismissed owing to the small size of the lesion as well as its subhepatic location. Chemotherapy could not be considered owing to the patient's comorbidities and renal failure, which made the use of cisplatin impossible.
In our experience, CT-guided percutaneous radiofrequency ablation could be an alternative to chemotherapy, either with or without surgery, for the treatment of local recurrence after nephroureterectomy for urothelial carcinoma of the upper urinary tract for patients in whom chemotherapy is contraindicated. Longer-term studies with a larger number of patients and follow-up are needed to confirm the utility of CT-guided percutaneous radiofrequency ablation.
Figures and Tables
FIG. 1
Computed tomography scan showing a 31-mm lesion with contrast uptake contiguous to the medial border of the right hepatic lobe (white arrow).
References
1. Jeldres C, Sun M, Isbarn H, Lughezzani G, Budaus L, Alasker A, et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. 2010; 75:315–320.
2. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998; 52:594–601.
3. Rassweiler JJ, Schulze M, Marrero R, Frede T, Palou Redorta J, Bassi P. Laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: is it better than open surgery? Eur Urol. 2004; 46:690–697.
4. Hattori R, Yoshino Y, Gotoh M, Katoh M, Kamihira O, Ono Y. Laparoscopic nephroureterectomy for transitional cell carcinoma of renal pelvis and ureter: Nagoya experience. Urology. 2006; 67:701–705.
5. Remzi M, Shariat S, Huebner W, Fajkovic H, Seitz C. Upper urinary tract urothelial carcinoma: what have we learned in the last 4 years? Ther Adv Urol. 2011; 3:69–80.
6. Childs MA, Wood CG, Spiess PE, Debiane LG, Hernandez M, Matin SF, et al. Early results of chemotherapy with retroperitoneal lymph node dissection for isolated retroperitoneal recurrence of upper urinary tract urothelial carcinoma after nephroureterectomy. Can J Urol. 2010; 17:5184–5189.
7. Sweeney P, Millikan R, Donat M, Wood CG, Radtke AS, Pettaway CA, et al. Is there a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J Urol. 2003; 169:2113–2117.
8. Abe T, Shinohara N, Harabayashi T, Sazawa A, Maruyama S, Suzuki S, et al. Impact of multimodal treatment on survival in patients with metastatic urothelial cancer. Eur Urol. 2007; 52:1106–1113.
9. Lehmann J, Suttmann H, Albers P, Volkmer B, Gschwend JE, Fechner G, et al. Surgery for metastatic urothelial carcinoma with curative intent: the German experience (AUO AB 30/05). Eur Urol. 2009; 55:1293–1299.
10. Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011; 77:1393–1397.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download