Abstract
Purpose
We hypothesized that there might be a higher incidence of low-risk prostate cancer (PCa) in men diagnosed at a repeated biopsy. Thus, we investigated differences in clinicopathological results of PCa after primary and repeated biopsy.
Materials and Methods
We retrospectively reviewed patients diagnosed with PCa at a primary or repeated biopsy from January 2004 to April 2011. Patients were stratified into primary biopsy and repeated biopsy groups. We analyzed prostate-specific antigen, clinical stage, Gleason score (GS), positive core ratio, and low-risk group by using D'Amico classification. We also investigated GS upgrading and upstaging after radical prostatectomy (RP).
Results
Among 448 primary and 37 repeated biopsy PCa patients, 82 (group 1) and 25 (group 2) underwent RP. The percentage of low-risk patients did not differ significantly between the groups. The positive biopsy core ratio was significantly lower in group 2 (p=0.009). The percentages of GS upgrading and upstaging were 42.7% and 47.6% in group 1, respectively (p=0.568), and 48.0% and 52.0% in group 2, respectively (p=0.901). In the analysis of low-risk patients, GS upgrading and upstaging were not significantly different between the groups (p=0.615 and p=0.959, respectively).
Conclusions
A lower positive core ratio may imply a small volume of PCa and possibly insignificant PCa in the repeated biopsy group. However, no significant differences were observed for the ratio of low-risk cancers, GS upgrading, or upstaging between the groups. Therefore, PCa diagnosed at a repeated biopsy is not an additional indication for active surveillance.
Active surveillance is an alternative management option in patients with low-risk prostate cancer and a favorable disease-specific prognosis. Several studies have shown no significant difference between the clinical results of a radical prostatectomy (RP) and that of active surveillance in men with low-risk prostate cancer [1]. Therefore, it is important to form an opinion of low-risk prostate cancers in advance.
Transrectal ultrasound (TRUS)-guided prostate biopsy is the standard diagnostic procedure for prostate cancer detection. About 20% to 40% of patients are diagnosed with prostate cancer at an initial biopsy, whereas patients with an initial negative biopsy result are typically followed with periodic serum prostate-specific antigen (PSA) measurements [2]. Persistent increases in serum PSA after an initial biopsy often drive the decision for repeat biopsy. Because prostate cancer is often multifocal and the prostate volume sampled during biopsy is relatively small, these individuals may have prostate cancer despite their initial biopsy results being negative [3]. Indeed, recent studies have shown that cancer is detected in 10% to 25% of these individuals on a repeat biopsy [4].
The impact of prostate cancer diagnosed after repeat biopsies on prognosis has been debated in a few reports. Recent studies have shown that prostate cancer diagnosed after repeat biopsies was related to better pathological outcomes. Numao et al. [5] reported that initial transrectal 12-core prostate biopsy-negative cancers had a significantly lower incidence of abnormal digital rectal examination (DRE) results and a higher incidence of a Gleason score (GS) ≤6 and a Gleason grade ≤3 than did initial biopsy-positive cancers. Park et al. [6] reported that, compared with patients diagnosed at an initial biopsy, those diagnosed at repeat biopsies were more likely to have a lower clinical stage (cT1c: 79.5% vs. 55.5%, p<0.001) and organ-confined tumors (78.3% vs. 61.3%, p=0.003).
However, other investigators have reported conflicting results on the same issue. For example, Khang et al. [7] noted that the pathological outcomes of prostate cancer detected at a repeat biopsy were not significantly different from those of prostate cancer detected at an initial biopsy, except for a lower (<10%) percentage tumor volume. Epstein et al. [8] suggested that prior benign biopsy in men subsequently diagnosed with prostate cancer does not indicate an indolent tumor, because only 28.4% of patients with a prior benign biopsy had a "very limited" tumor. Furthermore, of the prior benign biopsy cases, 12% had positive margins and 27% had nonorgan-confined disease [8]. Meanwhile, Tan et al. [9] reported that a significant number of high-grade prostate cancers were detected on the third or greater prostate biopsy, thus underscoring the importance of repeat prostate biopsy in the setting of increased or increasing PSA despite a previous negative prostate biopsy result.
Debate about the impact of prostate cancer diagnosed after repeat biopsies on prognosis is ongoing. We hypothesized that there might be a higher incidence of low-risk prostate cancer in men with prostate cancer diagnosed at a repeated biopsy. Thus, we investigated differences in clinical results between a primary biopsy group and a repeated biopsy group.
We retrospectively reviewed the medical records of patients seen from January 2004 to April 2011 at our institution. Several participants were excluded owing to missing documentation about the pathologic features of the initial biopsy, because these patients were referred from other clinics. Also, some patients were lost to follow-up after detection of prostate cancer at biopsy. Four hundred forty-eight patients were diagnosed with prostate cancer at the primary biopsy. Among them, 82 patients (group 1) underwent a radical prostatectomy. For the same period, 121 patients underwent repeated biopsy. Among them, 37 patients (30.5%) were diagnosed with prostate cancer and 25 patients (group 2) underwent a radical prostatectomy. Biopsy was repeated on the basis of clinician preference and the policy on PSA increases (PSA velocity >0.75 ng/mL/y) and/or abnormal DRE and/or hypoechoic lesion by TRUS, or prior atypical small acinar proliferation or high-grade prostatic intraepithelial neoplasia, but not on definite criteria.
TRUS-guided prostate biopsies were performed with an 18-gauge needle biopsy gun by two radiologists at our institutions. Prostate volume was measured by TRUS during the initial biopsy. The number of biopsy cores ranged from 6 to 12 and was not based on definite criteria. Blood specimens for PSA measurements were obtained before any prostatic manipulations, including DRE or TRUS. We used the 2002 American Joint Committee on Cancer TNM staging system, and pathologic findings were evaluated by one pathologist. We analyzed the clinicopathological characteristics of both groups, including age, serum PSA level, biopsy GS, number of cores taken, number of cores positive for prostate cancer, prostate volume, clinical stage based on DRE and imaging study (prostate magnetic resonance imaging), pathological stage, and pathological GS and classified the low-risk group by using D'Amico classification [10]. Low-risk disease was defined by a PSA level <10 ng/mL, biopsy GS ≤6, and clinical stage T1 or T2a [10]. We also investigated GS upgrade and upstaging after radical prostatectomy.
All statistical analyses were performed by using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Quantitative data are expressed as mean±standard deviation. The continuous variables were analyzed by Student t-test (two-tailed), and Fisher exact test (two-tailed) was used for categorical variables. A p-value <0.05 was considered significant for all analyses.
The clinicopathological characteristics of the two groups of patients are presented in Table 1. Patient age, body mass index, PSA, prostate volume, the positive ratio on a digital rectal exam, GS, and clinical stage were not significantly different between the groups. The positive biopsy core ratio was significantly lower in the repeated biopsy group than in the primary biopsy group (p=0.009), reflecting the lower cancer burden in the repeat biopsy group. In addition, the positive hypoechoic nodule ratio on TRUS was higher in the primary biopsy group than in the repeated biopsy group (p=0.001).
The GS upgrade ratio was 42.7% in group 1 and 48.0% in group 2, but the difference was not significant (p=0.568) (Table 2). The upstaging ratio after the operation was 47.6% in group 1 and 52.0% in group 2 (p=0.901) (Table 3). The rate of low-risk cancer was not significantly different between the repeat biopsy group and the primary biopsy group (20.0% vs. 23.2%, p=0.739) (Table 4).
In the subgroup analysis of 24 patients classified as low-risk before the operation, GS upgrading (group 1, 52.6%; group 2, 40%; p=0.615) and upstaging (group 1, 78.9%; group 2, 80%; p=0.959) were not significantly different between the groups (Table 4).
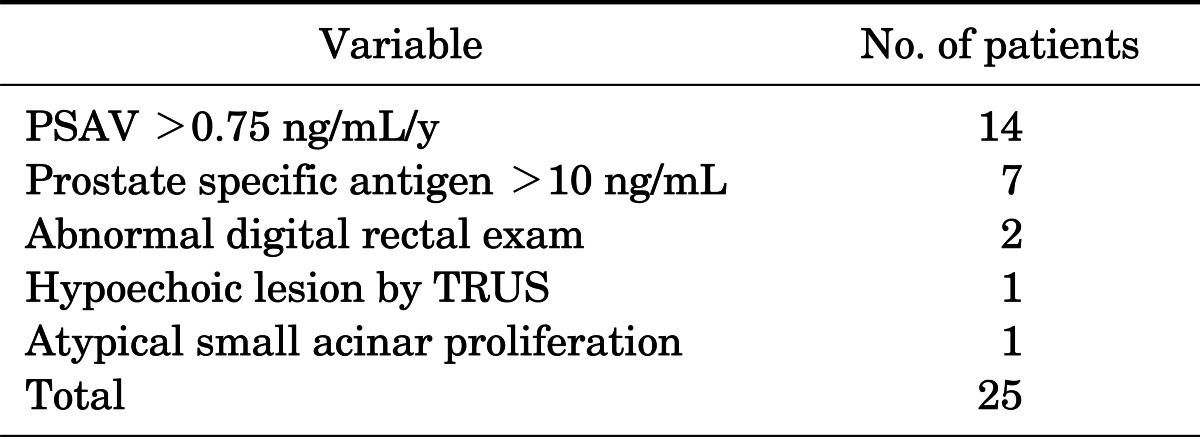
The reasons for rebiopsy were as follows: 1) elevated PSA velocity (0.75 ng/mL/y, n=14), 2) elevated PSA during follow-up (>10 ng/mL, n=7), 3) abnormal DRE (n=1), 4) hypoechoic lesion by TRUS (n=1), and 5) atypical small acinar proliferation (n=1) (Table 5).
As a consequence of the stage and grade migration of prostate cancer resulting from the widespread use of PSA testing, the notion of insignificant prostate cancer has emerged. Insignificant prostate cancer is defined as a low-grade, small-volume, organ-confined cancer that is unlikely to progress to a clinically and biologically significant tumor without treatment [11].
Active surveillance is an alternative management option in patients with low-risk prostate cancer. However, there are no definitive guidelines on whether to perform active surveillance in prostate cancer patients undergoing repeated biopsy but with negative findings on the initial prostate biopsy. We can expect that initial-biopsy-negative cancers will have a low cancer volume, because they escaped detection initially. It has been reported that prostate cancer diagnosed after repeat biopsy is related to a small cancer volume [6]. A lower cancer volume of prostate cancer diagnosed after repeat biopsies may lead to insignificant prostate cancer and classification in the low-risk group according to the D'Amico classification system [10]. Thus, we expected that prostate cancer diagnosed after repeat biopsies might be classified into the low-risk category and be more appropriate for active surveillance.
Our data showed that the positive biopsy core ratio was significantly lower in the repeated biopsy group. This implies a better prediction of insignificant prostate cancer in the repeated biopsy group. However, the three factors that determine the risk classification-PSA, GS, and clinical stage-were not significantly different. The updated Epstein criteria consist of PSA density ≤0.15 ng/mL/g, GS ≤6, fewer than three positive cores, and <50% of cancer involvement in any core. Updated Epstein criteria are used to predict small-volume prostate cancer (<0.5 cm3) [11]. So, a low positive biopsy core ratio may correlate with tumor volume. However, the Epstein criteria are not perfect and misclassify about 30% of patients [11]. These discrepancies can be explained by several factors, such as grade misclassification and number of cores obtained at biopsy.
Numao et al. [5] and Park et al. [6] have reported that prostate cancers diagnosed at repeat biopsies are more likely to have favorable data. However, our study reported conflicting results on the same issue. These discrepancies can be explained by the number of cores obtained at the initial biopsy. Villa et al. [12] explored whether limited biopsy strategies could adequately assess cancer risk. They identified 233 patients who underwent RP with minimal prostate cancer defined as a single core with a Gleason sum of 6 involving less than 5% or 0.5 mm in core length. The authors examined the RP specimens to determine how many had pathologically insignificant disease defined as a pathological GS of 6 or less, tumor volume of 0.5 mL or less, and organ-confined disease. Of note, the number of cores correlated with the incidence of pathologically insignificant cancer. The rate of pathologically confirmed insignificant prostate cancer was 3.8%, 29.6%, and 39.4% in patients who underwent biopsy of 12 or fewer cores, 13 to 18 cores, and 19 or more cores, respectively (p<0.001). Each additional core increased the risk of insignificant cancer on final pathology by 16% [12]. Thus, the number of cores should be taken into account.
We also evaluated whether initial transrectal 12-core prostate biopsy-negative cancers had a significantly lower incidence of abnormal DRE findings and a higher incidence of GS ≤6 and Gleason grade ≤3 than did initial biopsy-positive cancers [5]. Park et al. [6] reported that, compared with patients diagnosed at an initial biopsy, those diagnosed at repeat biopsies were more likely to have a lower clinical stage (cT1c: 79.5% vs. 55.5%, p<0.001) and organ-confined tumors (78.3% vs. 61.3%, p=0.003).
We also evaluated whether initial biopsy-negative cancers were associated with GS upgrading and pathological upstaging in patients who underwent radical prostatectomy, because the clinical significance of cancer detection on repeated biopsy was one of our objectives. In the present study, the positive hypoechoic nodule ratio on TRUS was higher in the primary biopsy group than in the repeated biopsy group, but the clinical stage was not different. Also, the upstaging ratio after the operation was not significantly different between the groups. These results indicate that cancer detected on a repeated biopsy is not pathologically and clinically different from cancer detected on a first biopsy.
In 2004, Bastian et al. [13] published an update of the preoperative Epstein criteria identifying pretreatment criteria significantly predictive of insignificant PC in RP specimens using PSA and needle biopsy findings. The updated criteria consist of PSA density ≤0.15 ng/mL2, GS ≤6, fewer than three positive cores, and <50% cancer involvement in any core. To date, these are the most widely used preoperative criteria for predicting insignificant prostate cancer. Most definitions of insignificant prostate cancer will result in low-risk disease at surgery, and the main aim of the insignificance definition is to avoid surgery or other treatment modalities. Although prior studies have shown that cancer identified on a repeat biopsy typically has a lower volume and carries less risk than cancer found during the initial prostate biopsy [8,14,15], our study revealed that a previous negative biopsy result is not a predictor of low-risk prostate cancer.
Patients with prior negative biopsy results are more likely to have a lower volume of cancer [6]. Nevertheless, our findings suggest that these patients are still at risk for high-grade, localized cancer in the highly selective setting of repeat biopsy for persistently suspicious findings after a prior negative biopsy.
The clinical implication of diligent follow-up in this setting is that these tumors may be significant and have a higher likelihood of cure on the basis of their size. Thus, detection may be particularly important in the setting of persistently increased or increasing PSA following a negative biopsy result, especially if only a single prior biopsy was performed. It is clear that if a clinical reason exists to suspect cancer, rebiopsy is certainly appropriate.
Our study had several limitations. First, this retrospective study could not avoid inherent biases. Second, the numbers of biopsy cores differed (6-12) for each period owing to a change in our medical center's biopsy protocols. Because biopsies with fewer than 12 cores result in a lower cancer detection rate than 12-core biopsies, variation in the number of biopsy cores could have affected the results. Third, our cohort was selected from patients who underwent RP rather than from the entire population of patients with biopsy-confirmed prostate cancer, which may have introduced a selection bias. Forth, we could not assess tumor volume in our cohort owing to a lack of pathologic data. Among the factors influencing the definition of insignificance, a methodologic confounder is how tumor volume is calculated. The assessment of tumor volume has been hampered by the lack of a convenient and accurate method for determining prostate cancer volume.
Despite these limitations, this is the first study reporting no significant differences in the ratio of low-risk cancers, GS upgrading, or upstaging between a primary biopsy group and a repeated biopsy group. Our recommendation to physicians is that cancer detected on a repeated biopsy is not favorable compared with the cancer detected on first biopsy. Thus, active treatments are needed for patients diagnosed with prostate cancer at a repeated biopsy.
The positive biopsy core ratio was significantly lower in the repeated biopsy group than in the primary biopsy group. This may imply a smaller-volume prostate cancer in the repeated biopsy group and could be one of the reasons patients in the repeated biopsy group had negative findings at the primary biopsy. However, no significant differences were observed for the ratio of low-risk cancers, GS upgrading, or upstaging between the groups. Therefore, patients diagnosed with prostate cancer at a repeated biopsy are not at lower risk.
References
1. Liu D, Lehmann HP, Frick KD, Carter HB. Active surveillance versus surgery for low risk prostate cancer: a clinical decision analysis. J Urol. 2012; 187:1241–1246. PMID: 22335873.

2. Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010; 46:3095–3101. PMID: 21047592.

3. Djavan B, Susani M, Bursa B, Basharkhah A, Simak R, Marberger M. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. 1999; 5:139–142. PMID: 10527256.
4. Yanke BV, Gonen M, Scardino PT, Kattan MW. Validation of a nomogram for predicting positive repeat biopsy for prostate cancer. J Urol. 2005; 173:421–424. PMID: 15643192.

5. Numao N, Kawakami S, Sakura M, Yoshida S, Koga F, Saito K, et al. Characteristics and clinical significance of prostate cancers missed by initial transrectal 12-core biopsy. BJU Int. 2012; 109:665–671. PMID: 21939488.

6. Park M, You D, Yoon JH, Jeong IG, Song C, Hong JH, et al. Does repeat biopsy affect the prognosis of patients with prostate cancer treated with radical prostatectomy? Analysis by the number of cores taken at initial biopsy. BJU Int. 2012; 109:1474–1479. PMID: 21933324.

7. Khang IH, Kim YB, Yang SO, Lee JK, Jung TY. Differences in postoperative pathological outcomes between prostate cancers diagnosed at initial and repeat biopsy. Korean J Urol. 2012; 53:531–535. PMID: 22949996.

8. Epstein JI, Walsh PC, Akingba G, Carter HB. The significance of prior benign needle biopsies in men subsequently diagnosed with prostate cancer. J Urol. 1999; 162:1649–1652. PMID: 10524890.

9. Tan N, Lane BR, Li J, Moussa AS, Soriano M, Jones JS. Prostate cancers diagnosed at repeat biopsy are smaller and less likely to be high grade. J Urol. 2008; 180:1325–1329. PMID: 18707706.

10. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974. PMID: 9749478.
11. Ploussard G, Epstein JI, Montironi R, Carroll PR, Wirth M, Grimm MO, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol. 2011; 60:291–303. PMID: 21601982.

12. Villa L, Capitanio U, Briganti A, Abdollah F, Suardi N, Salonia A, et al. The number of cores taken in patients diagnosed with a single microfocus at initial biopsy is a major predictor of insignificant prostate cancer. J Urol. 2013; 189:854–859. PMID: 23022004.

13. Bastian PJ, Mangold LA, Epstein JI, Partin AW. Characteristics of insignificant clinical T1c prostate tumors: a contemporary analysis. Cancer. 2004; 101:2001–2005. PMID: 15372478.
14. Lopez-Corona E, Ohori M, Wheeler TM, Reuter VE, Scardino PT, Kattan MW, et al. Prostate cancer diagnosed after repeat biopsies have a favorable pathological outcome but similar recurrence rate. J Urol. 2006; 175(3 Pt 1):923–927. PMID: 16469581.

15. Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001; 166:1679–1683. PMID: 11586201.

TABLE 3
Comparison of stage migration pattern between primary biopsy group and repeated biopsy group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download