Abstract
Since their introduction in 1996, tension-free midurethral slings (MUS) have been proven to have long-term efficacy and safety. They are considered the gold standard treatment of female stress urinary incontinence, especially in cases that are associated with urethral hypermobility. However, they are not free of complications and, although rare, some of these complications can be challenging for both patients and physicians. Some complications occur intraoperatively, whereas others appear in the early or late postoperative period. There is less controversy in the diagnosis and treatment of complications such as vaginal extrusion or urinary system erosion, whereas de novo voiding problems are at best not completely understood. Voiding dysfunction after MUS placement may vary in a wide range from urinary frequency or urgency to retention and is usually attributed to the obstructive or irritative effect of the sling. However, present urodynamic criteria for the diagnosis of female infravesical obstruction are not satisfactory, and the best management policy for de novo voiding dysfunction remains controversial. In the majority of cases, the diagnosis of obstruction leading to a urethral release surgery depends on a combination of several clinical findings. The timing of urethral release surgery varies depending on the preferences of the surgeon, and the outcome of this surgery is not always predictable. The purpose of this review was to assess the diagnosis and management of the immediate, short-term, and long-term complications of MUS in light of the current literature in an attempt to determine the best management policy.
After the first clinical application of the pubovaginal sling a century ago, multiple types of slings composed of different materials, such as autografts, allografts, xenografts, and synthetic material, have been tried for years with the hope of defining the ideal method of suspension. In nearly all of these techniques, the sling was classically placed at the level of the bladder neck. However, competing theories proposed by DeLancey [1], Petros and Ulmsten [2] underlined the common concept that strong supportive suburethral tissue was essential for maintaining an adequate continence mechanism in women and that the level of the midurethra rather than the bladder neck was important for preventing urinary incontinence under stress circumstances. Using these concepts, Ulmsten et al. [3] introduced the tension-free vaginal tape (TVT) procedure in 1996. Subsequent to the development and successful application of the TVT and other retropubic (RP) procedures, Delorme initially described a new method of tension-free midurethral tape referred to as the transobturator tape (TOT) in 2001 as a viable alternative for correction of female stress urinary incontinence (SUI) [4]. High success rates reported in mid- and long-term studies have popularized these procedures more and more, and tension-free midurethral tapes are now gradually becoming the new gold standard treatment for female SUI. The recent American Urologic Association guideline for the surgical management of female SUI concluded that synthetic midurethral sling (MUS) surgery is an appropriate treatment with similar efficacy and less morbidity than conventional nonmesh slings [5].
The U.S. Food and Drug Administration (FDA) issued a Public Health Notification in 2008 warning about serious complications associated with the transvaginal placement of mesh for both SUI and pelvic organ prolapse [6]. But in the Public Health Notification the FDA issued in 2011, there was a warning about the use of transvaginal mesh for pelvic organ prolapse surgery only and not for SUI surgery. The FDA also noted that any reduction or restriction of the use of the MUS would be a disservice to patients [7].
Hemorrhagic complications and urinary tract injury are reviewed in this section.
Hemorrhage is relatively rare. Hemorrhage, defined as blood loss greater than 200 mL or postoperative hematoma, occurs in approximately 2% of patients and can usually be managed by observation or local compression. A recent prospective randomized study comparing the adverse effects after RP and transobturator (TO) MUS surgery reported that intraoperative blood loss (more than 100 mL) was the second most common intraoperative complication in both surgery groups and occurred twice as frequently in the RP group [9]. Flock et al. [10] suggested that inserting Redon drains in patients with increasing bleeding during TVT was helpful in reducing the risk of retropubic hematoma. Major vessel injury and bowel injury are exceedingly rare and are found in 0.07% and 0.04% of cases, respectively [11]. If major vessel injury is suspected because of unstable hemodynamic findings, primary repair can be possible [12]. Persistent low abdominal pain resistant to analgesics postoperatively must raise the suspicion of bowel injury, especially in patients with a previous history of pelvic operations. Laparoscopy or laparotomy with subsequent repair of the perforation site will usually solve the problem [13]. Catastrophic complications do occur and may result in mortality, as seven reported deaths exist in the database from 1998 to 2005 [7].
Major vascular injury has not been reported in TO MUS procedures. Risks of major complications such as large vessel and bowel injury are thought to be less given the anatomy of TO MUS needle passage [14]. Obturator hematoma and intraoperative hemorrhage have rarely been reported in TO MUS procedures [11].
The bladder perforation rate was reported to be between 1.1% and 15% and ranged from 2.7% to 3.8% in two large national registries [15,16] of RP MUS procedures. The risk of complications was higher in centers where RP MUS was occasionally performed [16].
A recent review assessing the evidence on the effectiveness and complications of RP and TO procedures reported that bladder perforation was significantly more common in the RP group [17].
In a recent randomized clinical trial it was shown that the route of trocar insertion, whether top to bottom or bottom to top, did not affect the bladder perforation rates [18]. A recent prospective randomized study comparing adverse effects after RP and TO MUS surgery reported that intraoperative bladder perforation (5%) occurred exclusively in the RP group [9].
The importance of recognizing bladder perforation intraoperatively was emphasized by Cetinel et al. [19]. Reinsertion of the tape with 2 days of urethral catheterization postoperatively was the suggested treatment in this situation. Otherwise, open or endoscopic surgery would be needed later to remove the tape inside the bladder [19]. Interestingly, Cetinel et al. [19] reported that cystoscopic examination was normal in two patients with bladder perforation and the perforation was determined only by suprapubic incisional fluid leakage after the trocars were removed. A recent retrospective review of patients undergoing RP MUS placement complicated by a cystotomy during sling placement showed that the majority of subjects (20 of 25 of patients) experiencing a cystotomy were successfully discharged home the day of surgery without catheter drainage [20]. Bladder perforation is a known intraoperative complication that, if left unrecognized, can have significant morbidity. A case of unrecognized bladder perforation leading to severe progressive cellulitis and candidal infection was recently reported [21]. Those authors concluded that unrecognized bladder perforation and nonbacterial causes of infection should be considered in patients with severe progressing cellulitis despite broad-spectrum antibiotic coverage after RP MUS placement [21].
Although initial reports described the bladder perforation risk with the TO approach as being negligible, the risk is now identified in some studies. This risk is much more rare than it is in RP procedures [11], because the planned trocar path during TO sling placement does not enter the pelvis. Case reports of bladder and urethral injuries have led to the current recommendation for routine intraoperative urethrocystoscopy [22]. Some authors reported incidences of urethral injury that were not insignificant (1.1-2.5%) during their early experience with TO slings [23,24]. These authors pointed out that urethral injuries were eliminated in their series after inserting a finger into the vaginal dissection to protect or deflect the urethra while passing the trocar. Notably, both authors used the UraTape (Mentor-Porges, Le Plessis Robinson, France), which has been withdrawn from the market owing to unacceptably high erosion rates attributed to a silicone coating on the midportion of the sling [25]. Urethral injuries are now less frequent in TO MUS procedures.
Infectious complications; vaginal, urethral, and intravesical tape erosions; and different types of de novo voiding dysfunction (VD) will be discussed in this section.
Two cases of necrotizing infections after TVT placement have been reported [26,27]. It was speculated that the presence of localized infection and morbid obesity could be possible risk factors for the development of necrotizing infections. Intensive resuscitation was necessary in one of these cases to resolve the problem.
In a nationwide analysis of complications associated with TVT procedures, Kuuva and Nilsson [16] reported that 4.1%, 0.8%, and 0.7% of patients had urinary tract infection, wound infection of the abdominal incision, and defective healing of the vaginal incision, respectively.
Infection-related complications after the TOT procedure have included thigh abscesses requiring drainage and an infected obturator hematoma also requiring exploration and drainage [11].
Vaginal extrusion is a rare complication and its incidence is reported to range from 0.5% to 1.1% [9]. Some possible reasons for vaginal extrusion have been suggested to be wound infection, impaired wound healing, an improper vaginal dissection plane, and vaginal atrophy [28]. Symptoms of vaginal erosion include vaginal discharge, a palpable rough surface in the vagina, sexual discomfort (usually partner related), and lower urinary tract symptoms including hematuria. A high index of suspicion is required. The management options are not standardized and range from observation to partial and complete tape excision and reapproximation of the vaginal mucosa over the exposed tape. Initial observation for small vaginal erosions with the use of topical estrogen creams when appropriate is recommended. Excision should be reserved for failure of conservative treatment. Good results have been achieved with reapproximation of the vaginal mucosa. Even with partial excision of the tape, continence is maintained in the majority of patients.
Urethral erosion is defined as the presence of sling material within the urethral lumen. The incidence of urethral erosion is extremely low after RP slings. Excess tension of the sling on the urethra, and technical error during dissection of the plane beneath the urethra, resulting in compromised thickness of suburethral tissue, were stated as possible risk factors for urethral erosion [29]. The erosion could be a result of an unrecognized urethral perforation during the original procedure [30]. VD is predominant with symptoms of urgency, urgency incontinence, obstructive voiding, and sometimes urinary retention. Recurrent urinary tract infection may be seen. Diagnosis is made by determining the presence of the tape within the urethral lumen during cystoscopy. Conservative observational treatment is not an option. Endoscopic tape transection or transvaginal excision of the tape with closure of the urethrotomy is the treatment option. A Martius fat pad graft may be used in case of extensive urethrotomies. A sling preferentially autologous can be placed at the time of surgery or in a delayed stage to treat possible SUI.
Intravesical tape erosion is the finding of sling material within the lumen of the bladder and is exceedingly rare following the RP MUS procedure. The vast majority of this complication is due to inadvertent and unrecognized bladder perforation during the original procedure. Thus, complete and thorough cystoscopic evaluation by an experienced urologist is essential to minimize this complication. Typical symptoms are intermittent gross hematuria, pelvic pain, and storage-type lower urinary tract symptoms. Recurrent urinary tract infection after RP surgery must arouse the suspicion of this possibility. Observational treatment is not an option. The sling material inside the bladder must be removed either endoscopically or by open surgery. Only patients with complete tape removal have been reported to have recurrent stress incontinence [11].
Mesh-related complications (exposures and erosions) affected 3% to 5% of participants (RP 4.7% vs. TO 3.0%) in the TOMUS study [9].
The same possible reasons for vaginal and urethral erosions for RP MUS procedures are consistent for TO procedures as well. The intravesical tape erosion rate is expected to be lower than it is for RP procedures because bladder perforation rates are consistently and significantly lower in TO MUS procedures [31]. Domingo et al. [32], who used Obtape or Uratape as the sling material, reported a relatively high vaginal extrusion rate (15%). They attributed this high erosion rate to the reduced pore size of their mesh (50 µm). They concluded that synthetic mesh with larger pore sizes facilitated vascular and tissue ingrowth, thus optimizing mesh incorporation. Management options are the same as in RP slings.
The presenting symptoms of VD may vary in terms of type and severity in a range between urinary frequency and urinary retention. Patients may have complaints such as straining to void, incomplete emptying, urgency and frequency, urge incontinence, and total urinary retention. Elevated postvoid residual (PVR), low urine flow rates, and recurrent urinary tract infections may usually be determined. The pathophysiology of VD after MUS is not well understood, although it is assumed to be caused by urethral obstruction or irritation by the mesh in most cases [33]. Other causes such as bladder perforation, pelvic hematoma, urethral erosion, or vaginal extrusion of the mesh should always be considered in the differential diagnosis. Although obstruction appears to be the main etiological factor, there is not a precise method for diagnosing obstruction and predicting the patients who will benefit from a urethral release surgery [34,35].
Urinary retention stands at the edge of the symptom spectrum of VD seen after MUS. The definition of urinary retention after MUS varies between studies. It may be defined as catheter-dependency for at least 28 days [36]. There is no consensus about the cutoff level of PVR after voiding trials that necessitates catheterization. For a clinically significant PVR, some authors propose proportional definitions such as 20% to 50% of the bladder capacity, whereas others use clearly defined levels of PVR ranging from 100 to 150 mL [37]. The discomfort of the patient also plays an important role in the decision making for clean intermittent or indwelling catheterization. It is important to note that symptoms that persist beyond 4 weeks after sling surgery rarely resolve spontaneously [38].
Increased outlet resistance by the over-tensioned sling appears to be the main causative factor for urinary retention after MUS. Several authors have further suggested that preoperative urodynamic findings indicating relatively impaired detrusor contractility may predict postoperative retention. For example, Kleeman et al. [39] showed that a preoperatively high PVR was a significant risk factor in predicting postoperative retention after different types of anti-incontinence and prolapse surgeries. In another study, Miller et al. [40] found that no other preoperative urodynamic parameters but a detrusor pressure less than 12 cmH2O was significantly associated with urinary retention after pubovaginal sling surgery.
On the other hand, Hong et al. [41] showed a low preoperative urine flow rate to be the only predictive preoperative factor for postoperative retention after RP MUS. Although heterogeneous findings and disagreement do exist, impaired detrusor contractility may be a risk factor for urinary retention after sling surgery.
Urinary retention may first be recognized when the patient fails to empty her bladder at the first voiding trial after MUS surgery. The different methods of performing the first voiding trial can affect the incidence of retention. For example, Foster et al. [42] showed that after MUS, women are more likely to empty their bladders effectively before discharge if they are evaluated with a backfill-assisted voiding trial compared with spontaneous natural bladder filling and emptying. Kim et al. [43] further showed that postoperative VD is common in the early postoperative period but may be transient and associated with the immediate voiding conditions following surgery such as increased fluid load and bladder overdistention. The latter study showed that that even among patients who fail the initial voiding trial, 36.8% can successfully void on subsequent trials. On the other hand, Wheeler et al. [44] demonstrated that 16.4% of patients who pass the initial voiding trial may fail on the second. The aforementioned studies suggest that the bladder should not be overfilled for the first voiding trial and that approximately one-third of the women who fail an initial trial can successfully empty their bladders in subsequent trials.
When the patient cannot void after MUS surgery, many surgeons prefer indwelling bladder catheterization up to 1 week (3 to 7 days) and retesting the patient after catheter removal. There is, however, almost no consensus in the literature about the strategy to follow when the voiding trial 1 week after sling surgery fails. In cases of retention lasting longer than 1 week, some surgeons prefer an early surgical intervention to cut the tape, whereas some prefer to switch to clean intermittent catheterization as advocated by Elliott and Comiter [37]. However, there is a paradigm shift among surgeons toward earlier intervention because delayed time to urethrolysis and longstanding obstruction can lead to irreversible bladder dysfunction.
The presenting symptoms may include storage symptoms such as increased frequency, urinary urgency, nocturia, and urgency incontinence or emptying symptoms such as hesitancy, straining to void, weak urinary stream, incomplete emptying, and urinary retention. Patients may also present with bladder pain, dysuria, or urinary tract infections. The timing of symptoms is the best diagnostic parameter for understanding the etiology. Symptoms that were not present preoperatively but appear after a MUS surgery should be considered as mesh- or surgery-related. Supporting that view, Patel et al. [36] indicated "the temporal relationship between the sling procedure and onset of symptoms" as the single most important factor in the diagnosis of sling-related obstruction or VD. However, it should also be kept in mind that VD may develop insidiously, and in these cases the dysfunction may not be easily related to the previous MUS surgery. For example, Carr and Webster [34] reported that in women with postoperative VD following prior incontinence surgery, 12% of the women described a gradual onset of symptoms as remote as 1 year or greater. It has also been suggested that mild symptoms after MUS are underdiagnosed and underreported, which in part may play a role in cases of insidious onset [45]. A high index of suspicion is certainly needed in cases with insidious onset of symptoms following MUS surgery.
Physical examination of a patient with VD after MUS surgery should include a basic pelvic inspection with the evaluation of vulva and vaginal introitus, vaginal canal, and urethra, together with the assessment of urethral mobility and pelvic organ prolapse. Vaginal extrusion of the mesh or a significant prolapse that bends the vesico-urethral angle causing obstruction should be ruled out. Vaginal examination may also reveal overcorrection of the urethral axis with bladder neck or midurethral kinking. A stress test should also be applied to rule out persisting SUI. In patients who present with neurological symptoms, dyspareunia, or pain, the physician should try to locate the origin of the pain. It should be remembered that neurologic symptoms might occur in groin areas or in suprapubic areas after RP MUS and TO MUS, respectively [9]. Persistent leg pain should raise the suspicion of urethral erosion or vaginal extrusion [46]. An undiagnosed bladder perforation or pelvic hematoma may cause bladder irritability and de novo urgency that is more common after RP MUS [47]. Bladder perforation after TO MUS is relatively uncommon but still possible [48,49]. Voiding cystourethrograms may be better combined with urodynamic studies and can provide a better assessment of mesh-related urethral obstruction.
Cystoscopy should be reserved for patients with hematuria, bladder pain, or recurrent cystitis, especially when bladder perforation or urethral mesh erosion is suspected. Urethrocystoscopy is indicated to rule out urethral kinking (abnormally vertical urethral axis) in addition to evaluating for sling erosion into the urethra or bladder. Many authors suggest that cystoscopy be routinely performed at the time of a urethrolysis operation to rule out intraurethrovesical problems.
Urodynamic evaluation of a patient with VD after MUS may include noninvasive tests such as PVR measurement and uroflowmetry and invasive tests such as cystometry and pressure flow studies.
Bladder outlet obstruction in men is defined by the presence of a high pressure and low flow micturition revealed by pressure flow studies [50], whereas diagnosis of obstruction in women lacks well-defined urodynamic criteria. Anatomical differences of the female pelvis allow women to empty their bladders just by relaxing the pelvic floor, sometimes with additional help from the abdominal muscles without a strong detrusor contraction compared with men [51]. Therefore, even small changes in detrusor pressure during voiding may define female infravesical obstruction and it is therefore impossible to develop reliable diagnostic nomograms as we have for men. Instead, it may be a better strategy to compare the preoperative and postoperative urodynamic studies in the case of VD after MUS to delineate the effect of MUS surgery on micturition. However, the weak recommendation for invasive urodynamic study for an index patient with SUI prior to MUS surgery leads to lesser utilization of preoperative urodynamic study [52]. Thus, in today's practice, preoperative urodynamic data may not be present for comparison in most cases.
In an attempt to diagnose female bladder outlet obstruction, several authors have proposed different urodynamic criteria. In 1998, Chassange et al. [53] defined obstruction by using cutoff values for Maximum flow rate and Detrusor pressure at maximum flow rate. They indicated a Qmax of 15 mL/s or less combined with pdetQmax of 15 cmH2O or more to have a sensitivity of 80% and a specificity of 83.1% for diagnosing obstruction. The same group revised their cutoff values in 2000 and later in 2004, first by using women with SUI and then by using asymptomatic women as controls [54,55]. In the latter study, the highest sensitivity and specificity for predicting obstruction were at Qmax 12 mL/s or less and pdetQmax 25 cmH2O or greater. In another study, Nitti et al. [56] proposed criteria for video urodynamic study where obstruction was defined as radiographic evidence of obstruction between the bladder neck and the distal urethra in the presence of a sustained detrusor contraction of any magnitude during voiding. Blaivas and Groutz [57] designed a nomogram based on noninvasive Qmax and pdetmax. In 2006, Akikwala et al. [51] compared these 5 contemporary urodynamic definitions for female bladder outlet obstruction (BOO) in 154 women who underwent videourodynamics to assess their correlation with each other and with clinical suspicion of BOO. They concluded that each urodynamic definition of female BOO had merit, whereas video-urodynamic criteria and 1998 cutoff point criteria had the highest concordance. The Blaivas-Groutz nomogram was found to overestimate obstruction compared with the other criteria, and Akikwala et al suggested it not be used as the sole or standard definition of obstruction in women [51].
The final goal of pressure flow studies is to differentiate patients who will benefit from urethrolysis. However, for many authors, the only absolute selection criterion for offering urethrolysis is a clear temporal relationship of symptoms to surgery, because PFS may not show classic obstructive voiding in women who benefit from urethrolysis [45]. Car and Webster [34] have shown that there were no predicting factors including urodynamics to show which patients would benefit from urethrolysis. Petrou et al. [35] demonstrated that ureterolysis outcomes were not different in patients with urodynamically proven obstruction and in patients who had surgery solely on the basis of clinical criteria. As a result of these studies it was suggested that the temporal relationship correlating symptoms with an antecedent surgical procedure should be the primary criterion in selecting patients for urethrolysis and tape release [11].
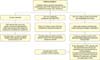
There are certain factors to be discussed regarding the diagnostic role of PFS. First, studies utilizing cutoff points derived their results from patients with clinical obstruction at the onset and not from women with functional obstruction. Furthermore, because obstruction is usually seen with overactive bladder symptoms, it is sometimes difficult to demonstrate obstruction urodynamically, especially if there are severe coexisting uncontrolled detrusor contractions. As a result, it is sometimes difficult to differentiate urethral obstruction from de novo urgency without obstruction, if it really exists! It is also important to note that about one-third of women cannot void in the presence of a cystometry catheter in PFS. Last, are there really cases in which obstruction is suspected clinically but clearly ruled out by urodynamics and vice versa? We certainly need further studies in this area to answer these questions. For these reasons, in most cases, persistent postoperative VD after MUS is initially treated conservatively, usually on a symptom basis. Temporary catheterization (indwelling or intermittent), pelvic floor muscle training (with or without biofeedback), and antimuscarinic drugs are the treatment options used in this stage. When these conservative methods fail, surgical treatment options such as transvaginal tape release with or without urethrolysis and segmental tape excision are usually indicated. Fig. 1 demonstrates an algorithm for the management of de novo VD after MUS according to the findings of our review of the literature.
A recent study evaluating the long-term results of TVT reported de novo overactive bladder symptoms in 30.1% and 18.9% of patients at 3 months and 10 years of follow-up, respectively [58]. A recent review assessing the evidence on the complications of RP and TO procedures revealed that the rates of de novo urgency were comparable in both procedures [17].
Clinical symptoms of urethral obstruction after RP MUS procedures have been reported with an incidence of 1.9% to 9.9% [11]. When the literature is reviewed regarding the surgical management of VD after RP MUS surgery, it is evident that midline transvaginal tape cut without urethrolysis is the most often performed surgical treatment and this treatment provides resolution of symptoms with maintenance of continence in the majority of patients [11]. Because recurrent SUI is most often seen in patients with tape cut in the very early postoperative period, a waiting period of at least 14 days but optimally 4 weeks before tape cutting is recommended [11,59].
The incidence of postoperative VD varies between 2.1% and 6.7% after TO MUS [11]. De novo urgency and incontinence were reported to be very rare in the TO and RP groups of the TOMUS study [9]. Regarding this complication, there appears to be no significant difference between RP and TO MUS in nonrandomized studies [20]. After TO sling surgery, obstructive urinary symptoms are usually transient and are usually managed by short-term intermittent catheterization, although occasionally symptoms do not subside, thus making tape release mandatory. A recent Cochrane review of MUSs concluded that TO slings are associated with less postoperative VD than are RP slings [60]. Management options are the same as in RP slings.
In a meta-analysis, postoperative urinary retention was found to be slightly more common in women undergoing RP MUS than in those undergoing TO MUS [17]. Brubaker et al. [9] also reported that VD requiring surgery (and/or catheter use) was more common after RP MUS than after TO MUS. Houwing et al. [61] compared VD rates and the need for reoperation between patients undergoing MUS procedures alone versus those having MUS procedures with concomitant prolapse repair. They found that RP or TO MUS with concomitant prolapse repair had a higher incidence of VD in the immediate postoperative period. However, this difference did not persist to the 6-week follow-up visit. The authors concluded that there was no greater risk of lasting VD or need for reoperation after concomitant procedures.
A bothersome complication unique to the TO MUS procedure is that of postoperative leg pain. Transient groin pain has been reported to occur with an incidence of 2.3% to 15.9%. This pain complication generally responds to nonsteroidal anti-inflammatories. The etiology of the pain is hypothesized to be due to either subclinical hematoma or a transient neuropathic phenomenon [11]. Persistent leg pain should raise the suspicion of urethral erosion or vaginal extrusion [46].
A recent review assessing the evidence on the complications of RP and TO procedures revealed groin or thigh pain to be significantly more common in the TO group [14].
Neurologic adverse effects such as numbness or weakness in the legs or pelvic area were reported to be more common in the TO group of the TOMUS study [9].
In a retrospective study comparing the late complications seen after either RP or TO slings, persistent pain was reported to be more common in the TO group (32%) than in the RP group (10%). In addition, dyspareunia was seen in 3.8% of women in the RP group versus 18.5% in the TO group [62].
The intraoperative complications of MUS procedures are rare and can be further minimized with a high index of suspicion and increased experience of the surgeon. Postoperative complications that vary from mesh erosion or extrusion to urinary retention are related to the presence of the mesh. Therefore, a safer mesh material for MUS surgery is certainly needed. The management of cases that are obviously obstructed (i.e., urinary retention, de novo emptying difficulty) is less controversial compared with de novo urgency, which is poorly understood. TO procedures seem to cause less de novo VD but more neurological symptoms than do RP procedures. We recommend that patients be thoroughly informed preoperatively about the possible complications of MUS surgery.
Figures and Tables
References
1. DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994; 170:1713–1720.
2. Petros PE, Ulmsten UI. An integral theory of female urinary incontinence: experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990; 153:7–31.
3. Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996; 7:81–85.
4. Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol. 2001; 11:1306–1313.
5. Dmochowski RR, Blaivas JM, Gormley EA, Juma S, Karram MM, Lightner DJ, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010; 183:1906–1914.
6. FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. October 20, 2008 [Internet]. Silver Spring: U.S. Food and Drug Administration;c2013. updated Mar 21 2013. cited Jul 1 2013. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm.
7. FDA Safety Communication: UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse. July 13, 2013 [Internet]. Silver Spring: U.S. Food and Drug Administration;c2013. updated Mar 21 2013. cited Jul 1 2013. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
8. Smith AR, Dmochowski R, Hilton P. Surgery for urinary incontinence. In : Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4th ed. Plymouth: Health Publication Ltd;2009. p. 1191–1272.
9. Brubaker L, Norton PA, Albo ME, Chai TC, Dandreo KJ, Lloyd KL, et al. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011; 205:498.
10. Flock F, Reich A, Muche R, Kreienberg R, Reister F. Hemorrhagic complications associated with tension-free vaginal tape procedure. Obstet Gynecol. 2004; 104(5 Pt 1):989–994.
11. Dmochowski R, Scarpero H, Starkman J. Tension-free vaginal tape procedures. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders;2007. p. 2251–2272.
12. Sivanesan K, Abdel-Fattah M, Ghani R. External iliac artery injury during insertion of tension-free vaginal tape: a case report and literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:1105–1108.
13. Meschia M, Busacca M, Pifarotti P, De Marinis S. Bowel perforation during insertion of tension-free vaginal tape (TVT). Int Urogynecol J Pelvic Floor Dysfunct. 2002; 13:263–265.
14. Moore RD, Gamble K, Miklos JR. Tension-free vaginal tape sling for recurrent stress incontinence after transobturator tape sling failure. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:309–313.
15. Cetinel B, Demirkesen O. Risk factors influencing the complication rates of tension-free vaginal tape-type procedures. Curr Opin Obstet Gynecol. 2005; 17:530–534.
16. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Acta Obstet Gynecol Scand. 2002; 81:72–77.
17. Long CY, Hsu CS, Wu MP, Liu CM, Wang TN, Tsai EM. Comparison of tension-free vaginal tape and transobturator tape procedure for the treatment of stress urinary incontinence. Curr Opin Obstet Gynecol. 2009; 21:342–347.
18. Andonian S, Chen T, St-Denis B, Corcos J. Randomized clinical trial comparing suprapubic arch sling (SPARC) and tension-free vaginal tape (TVT): one-year results. Eur Urol. 2005; 47:537–541.
19. Cetinel B, Demirkesen O, Onal B, Akkus E, Alan C, Can G. Are there any factors predicting the cure and complication rates of tension-free vaginal tape? Int Urogynecol J Pelvic Floor Dysfunct. 2004; 15:188–193.
20. Crosby EC, Vilasagar S, Duecy EE, Flynn MK. Expectant management of cystotomy at the time of midurethral sling placement: a retrospective case series. Int Urogynecol J. 2013; 24:1543–1546.
21. Fenderson JL, Washington BB, Hampton BS, Myers D. Unrecognized bladder perforation leading to severe progressive cellulitis and candidal infection. Female Pelvic Med Reconstr Surg. 2012; 18:130–131.
22. Minaglia S, Ozel B, Klutke C, Ballard C, Klutke J. Bladder injury during transobturator sling. Urology. 2004; 64:376–377.
23. Shepherd JP, Rapkin RB, Zyczynski HM. Urethral injury with transobturator midurethral sling. Female Pelvic Med Reconstr Surg. 2012; 18:132–133.
24. Costa P, Grise P, Droupy S, Monneins F, Assenmacher C, Ballanger P, et al. Surgical treatment of female stress urinary incontinence with a trans-obturator-tape (T.O.T.) Uratape: short term results of a prospective multicentric study. Eur Urol. 2004; 46:102–106.
25. Roumeguere T, Quackels T, Bollens R, de Groote A, Zlotta A, Bossche MV, et al. Trans-obturator vaginal tape (TOT) for female stress incontinence: one year follow-up in 120 patients. Eur Urol. 2005; 48:805–809.
26. Johnson DW, ElHajj M, OBrien-Best EL, Miller HJ, Fine PM. Necrotizing fasciitis after tension-free vaginal tape (TVT) placement. Int Urogynecol J Pelvic Floor Dysfunct. 2003; 14:291–293.
27. Connolly TP. Necrotizing surgical site infection after tension-free vaginal tape. Obstet Gynecol. 2004; 104:1275–1276.
28. Tsivian A, Kessler O, Mogutin B, Rosenthal J, Korczak D, Levin S, et al. Tape related complications of the tension-free vaginal tape procedure. J Urol. 2004; 171(2 Pt 1):762–764.
29. Kobashi KC, Govier FE. Management of vaginal erosion of polypropylene mesh slings. J Urol. 2003; 169:2242–2243.
30. Wai CY, Atnip SD, Williams KN, Schaffer JI. Urethral erosion of tension-free vaginal tape presenting as recurrent stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004; 15:353–355.
31. Latthe PM, Foon R, Toozs-Hobson P. Transobturator and retropubic tape procedures in stress urinary incontinence: a systematic review and meta-analysis of effectiveness and complications. BJOG. 2007; 114:522–531.
32. Domingo S, Alama P, Ruiz N, Perales A, Pellicer A. Diagnosis, management and prognosis of vaginal erosion after transobturator suburethral tape procedure using a nonwoven thermally bonded polypropylene mesh. J Urol. 2005; 173:1627–1630.
33. Nitti VW, Carlson KV, Blaivas JG, Dmochowski RR. Early results of pubovaginal sling lysis by midline sling incision. Urology. 2002; 59:47–51.
34. Carr LK, Webster GD. Voiding dysfunction following incontinence surgery: diagnosis and treatment with retropubic or vaginal urethrolysis. J Urol. 1997; 157:821–823.
35. Petrou SP, Brown JA, Blaivas JG. Suprameatal transvaginal urethrolysis. J Urol. 1999; 161:1268–1271.
36. Patel BN, Kobashi KC, Staskin D. Iatrogenic obstruction after sling surgery. Nat Rev Urol. 2012; 06. 05. [Epub]. http://dx.doi.org/10.1038/nrurol.2012.110.
37. Elliott CS, Comiter CV. Evaluation and management of urinary retention and voiding dysfunction after sling surgery for female stress urinary incontinence. Curr Bladder Dysfunct Rep. 2012; 7:268–274.
38. Rosenblum N, Nitti VW. Post-urethral suspension obstruction. Curr Opin Urol. 2001; 11:411–416.
39. Kleeman S, Goldwasser S, Vassallo B, Karram M. Predicting postoperative voiding efficiency after operation for incontinence and prolapse. Am J Obstet Gynecol. 2002; 187:49–52.
40. Miller EA, Amundsen CL, Toh KL, Flynn BJ, Webster GD. Preoperative urodynamic evaluation may predict voiding dysfunction in women undergoing pubovaginal sling. J Urol. 2003; 169:2234–2237.
41. Hong B, Park S, Kim HS, Choo MS. Factors predictive of urinary retention after a tension-free vaginal tape procedure for female stress urinary incontinence. J Urol. 2003; 170:852–856.
42. Foster RT Sr, Borawski KM, South MM, Weidner AC, Webster GD, Amundsen CL. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007; 197:627.e1–627.e4.
43. Kim JW, Moon du G, Shin JH, Bae JH, Lee JG, Oh MM. Predictors of Voiding Dysfunction after Mid-urethral Sling Surgery for Stress Urinary Incontinence. Int Neurourol J. 2012; 16:30–36.
44. Wheeler TL 2nd, Richter HE, Greer WJ, Bowling CB, Redden DT, Varner RE. Predictors of success with postoperative voiding trials after a mid urethral sling procedure. J Urol. 2008; 179:600–604.
45. Deng DY, Rutman M, Raz S, Rodriguez LV. Presentation and management of major complications of midurethral slings: are complications under-reported? Neurourol Urodyn. 2007; 26:46–52.
46. Mahajan ST, Kenton K, Bova DA, Brubaker L. Transobturator tape erosion associated with leg pain. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:66–68.
47. Daneshgari F, Kong W, Swartz M. Complications of mid urethral slings: important outcomes for future clinical trials. J Urol. 2008; 180:1890–1897.
48. Barber MD, Gustilo-Ashby AM, Chen CC, Kaplan P, Paraiso MF, Walters MD. Perioperative complications and adverse events of the MONARC transobturator tape, compared with the tension-free vaginal tape. Am J Obstet Gynecol. 2006; 195:1820–1825.
49. Abdel-Fattah M, Ramsay I, Pringle S. Lower urinary tract injuries after transobturator tape insertion by different routes: a large retrospective study. BJOG. 2006; 113:1377–1381.
50. Griffiths D, Hofner K, van Mastrigt R, Rollema HJ, Spangberg A, Gleason D. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction: International Continence Society Subcommittee on Standardization of Terminology of Pressure-Flow Studies. Neurourol Urodyn. 1997; 16:1–18.
51. Akikwala TV, Fleischman N, Nitti VW. Comparison of diagnostic criteria for female bladder outlet obstruction. J Urol. 2006; 176:2093–2097.
52. Nager CW, Brubaker L, Litman HJ, Zyczynski HM, Varner RE, Amundsen C, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012; 366:1987–1997.
53. Chassagne S, Bernier PA, Haab F, Roehrborn CG, Reisch JS, Zimmern PE. Proposed cutoff values to define bladder outlet obstruction in women. Urology. 1998; 51:408–411.
54. Lemack GE, Zimmern PE. Pressure flow analysis may aid in identifying women with outflow obstruction. J Urol. 2000; 163:1823–1828.
55. Defreitas GA, Zimmern PE, Lemack GE, Shariat SF. Refining diagnosis of anatomic female bladder outlet obstruction: comparison of pressure-flow study parameters in clinically obstructed women with those of normal controls. Urology. 2004; 64:675–679.
56. Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol. 1999; 161:1535–1540.
57. Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol Urodyn. 2000; 19:553–564.
58. Serati M, Ghezzi F, Cattoni E, Braga A, Siesto G, Torella M, et al. Tension-free vaginal tape for the treatment of urodynamic stress incontinence: efficacy and adverse effects at 10-year follow-up. Eur Urol. 2012; 61:939–946.
59. Long CY, Lo TS, Liu CM, Hsu SC, Chang Y, Tsai EM. Lateral excision of tension-free vaginal tape for the treatment of iatrogenic urethral obstruction. Obstet Gynecol. 2004; 104:1270–1274.
60. Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn. 2011; 30:284–291.
61. Houwing MM, Schulz JA, Flood CG, Baydock S, Rosychuk RJ. A retrospective review of tension-free vaginal tape/transobturator tape procedures done concomitantly with prolapse repair. J Obstet Gynaecol Can. 2013; 35:340–347.
62. Petri E, Ashok K. Comparison of late complications of retropubic and transobturator slings in stress urinary incontinence. Int Urogynecol J. 2012; 23:321–325.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download