Abstract
Purpose
To investigate the incidence of bladder cancer (BC) in Sri Lanka and to compare risk factors and outcomes with those of other South Asian nations and South Asian migrants to the United Kingdom (UK) and the United States (US).
Materials and Methods
The incidence of BC in Sri Lanka was examined by using two separate cancer registry databases over a 5-year period. Smoking rates were compiled by using a population-based survey from 2001 to 2009 and the relative risk was calculated by using published data.
Results
A total of 637 new cases of BC were diagnosed over the 5-year period. Sri Lankan BC incidence increased from 1985 but remained low (1.36 and 0.3 per 100,000 in males and females) and was similar to the incidence in other South Asian countries. The incidence was lower, however, than in migrant populations in the US and the UK. In densely populated districts of Sri Lanka, these rates almost doubled. Urothelial carcinoma accounted for 72%. The prevalence of male smokers in Sri Lanka was 39%, whereas Pakistan had higher smoking rates with a 6-fold increase in BC.
Conclusions
Sri Lankan BC incidence was low, similar to other South Asian countries (apart from Pakistan), but the actual incidence is likely higher than the cancer registry rates. Smoking is likely to be the main risk factor for BC. Possible under-reporting in rural areas could account for the low rates of BC in Sri Lanka. Any genetic or environmental protective effects of BC in South Asians seem to be lost on migration to the UK or the US and with higher levels of smoking, as seen in Pakistan.
Bladder cancer (BC) is the sixth most common cancer in the world, with an estimated 294,345 men and 88,315 women diagnosed with the disease and with 5-year survival rates varying from 40 to 80% [1,2]. In South Asia, the reported rate of BC is estimated to be about 2.1 per 100,000 [1], but there is currently no accurate estimate of the incidence of BC in Sri Lanka.
Age and male gender are known risk factors for BC, whereas other environmental risk factors include smoking and exposure to organic compounds such as the aromatic amines beta-naphthalene, benzidine, and 4-aminobiphenyl [3].
Transitional cell carcinomas (TCCs) are the most common type of BC, accounting for 95 to 97% in Europe [4]. In some African and Middle Eastern countries, squamous cell carcinoma is much more common as the result of chronic schistosomiasis infection [4].
We aimed to investigate the rate of BC in Sri Lanka by using the cancer registry data and to compare this with incidences in the neighboring countries of India and Pakistan as well as in South Asian migratory populations in the United Kingdom (UK) and the United States (US). The effects of smoking were analyzed to identify any effect on BC rates in Sri Lanka.
Sri Lanka, an island nation adjacent to the Indian subcontinent, has a population of 19 million people with Colombo, Gampaha, Kalutara, and Kandy districts having the highest population densities. Sri Lanka has a free public health care system similar to that of the National Health Service in the UK. In 2004 the Sri Lankan public health sector had 10,479 physicians with 6 physicians and 29 hospital beds per 10,000 persons, [5] which is similar to the public health systems of India (8 physicians per 10,000) and Pakistan (6 physicians per 10,000) [5]. The Sri Lankan public health care system serves the majority of the population, whereas the private health care system caters to a minority in the more developed cities.
The National Cancer Control Programme (NCCP) is the central collection agency for cancer data in Sri Lanka. New diagnoses of BC are recorded by the NCCP, who analyze, verify the diagnosis, and follow up records for 2 to 3 years from the diagnosis. The NCCP continuously monitors the data for any discrepancies and re-verifies any inconsistencies by tracing the initial source to ensure reliability. These data are used to build up the national cancer registry. The BC data (ICD-10 C67) supplied to us were verified by the NCCP and were collected immediately prior to the publication. More than 80% of the data are based on histological diagnoses (personal communication). To ensure reliability, we also analyzed BCs from the histopathological laboratories of the National Hospital of Sri Lanka (NHSL) for 2009 and 2010. The histopathological laboratories receive all specimens from the NHSL Urology wards, which admits patients mainly from the western province of Sri Lanka (comprising of Colombo, Gampaha, and Kalutara districts), and are analyzed and reported by consultant pathologists.
Smoking rates in Sri Lanka were provided to us by the Alcohol and Drug Information Center (ADIC) Sri Lanka. ADIC Sri Lanka conducts a bi-annual "spot" survey in 1,500 males aged 15 years and over by means of a questionnaire over a 2-week period and is carried out in the 4 most populated districts of Sri Lanka as well as in Anuradhapura (a rural district) and a randomly selected district. These smoking rates were analyzed and compared with those of other South Asian countries as well as the migrant population of the UK and the US.
The age-specific and age-standardized incidences of BC in Sri Lanka were calculated by using 5-year age bands for the period 2001 to 2005 with use of the 2001 national consensus data (estimated growth rate according to World Bank development indicators [6]) and were standardized by using the hypothetical Segi's world population, which was devised by cancer epidemiologist Segi [7]. Separate gender-specific rates and rates for the main cities were calculated by using the methods described above.
There were 514 new cases of BC in males and 123 in females over the 5-year period of analysis of an estimated 9,247,283 males and 9,433,225 females (between 83 and 118 per year in males and between 15 and 34 per year in females) with mean ages of 64 and 62 years at diagnosis, respectively. The overall incidence of BC in Sri Lanka remained low at 0.8 per 100,000; however, the age-adjusted rate of BC has increased since 1985.
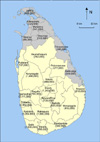
There was no accurate population consensus in the north and eastern parts of the country (Jaffna, Mannar, Vavuniya, Mullaitivu, Killinochchi, Batticaloa, and Trincomalee districts) owing to the civil war in those areas at the time. Furthermore, these areas accounted for only 10 male cases and 7 female cases in the 5 years. Therefore, these areas were excluded and the data from the other 18 districts were analyzed (Fig. 1).
The highest rates of BC were seen in the 4 most populated districts, of which Colombo, the most populated district, had the highest rates (2.17 per 100,000 in males and 0.62 per 100,000 in females) (Fig. 2).
The incidence of BC in Sri Lanka increased with age and with peak rates in males between 70 and 74 years of age (14.24 per 100,000) and in females aged 75 years and older (2.32 per 100,000) (Fig. 3).
Of the BCs in Sri Lanka, 72% were TCCs, with a male-to-female ratio of 4:1. The rest of the tumors were categorized as squamous cell carcinoma (9%), adenocarcinoma (3%), "other" (7%), and "unspecified malignant neoplasms of the bladder" (9%). Squamous cell carcinomas (11%) and adenocarcinomas (4%) were found more frequently in females than in males (9% and 3%, respectively).
Although a significant proportion of the TCC staging data was missing (62%), of the available data, 94% (158 out of 167) were pT2 or greater at presentation, which suggested that most tumors presented at an advanced stage. In keeping with the NCCP cancer registry database, the NHSL data for Colombo had 70 new diagnoses of BC over the 2-year period with a mean age of 65.4 years. Of these patients, 84.3% were male and TCCs accounted for 92.9% of BC with 45.7% of these being high grade and but only 27.1% being pT2.
On average, 38.6% of Sri Lankan males are reported as being smokers with the highest rates seen in the 25- to 39-year-old age group. Interestingly, the smoking rates in the highly populated districts were lower than in the rural districts (34.2% in Colombo vs. 43.1 % in Anuradhapura).
BC was the 15th most common cancer in Sri Lanka in 2000 [8]. During the 5-year period of analysis, the cancer registry data showed an overall increase in the rate of BC in Sri Lanka but this was lower than the Globocan estimates (1.36 and 0.3 per 100,000 vs. 2.2 and 0.5 per 100,000) [1]. However, in the analysis of individual district-level data, Colombo, which had the highest population and better health care access and data collection, showed an incidence of BC in both males and females that was higher than the Globocan estimated incidence (Fig. 2) [1]. Although is it possible that increasing BC rates in Sri Lanka could actually be due to improvements in data collection rather than to a true increase in incidence, it is evident that BCs in the rural districts present late as seen by the higher proportion of BCs that were pT2 or greater compared with the cases of Colombo. Therefore, it is also likely that the incidence of BC, especially in the rural districts, could be underreported and that the rates in Colombo reflect the true incidence of BC in Sri Lanka.
Although the incidence of BC in Sri Lanka was lower than the incidences in India and Pakistan, the rates in Colombo were comparable with those of India and Bangladesh (Fig. 2) [1,9,10]. Surprisingly, Pakistan had the highest incidence of BC among the South Asian countries (Fig. 1) [10].
The majority (72%) of the BCs in Sri Lanka were TCCs, which was lower than the NHSL data, the rates in other South Asian countries, and previously reported rates (93.4%) [11]. The significant number of tumors categorized as "unspecified carcinomas" in the cancer registry data, better hospital access, and data collection influencing the NHSL data could explain these differences. Squamous cell BCs are commonly associated with schistosomiasis infection, as seen in Egypt [2]. Although these accounted for 9% of the cancer registry BCs, the reported schistosomias infections have been limited to a few case reports in India, Pakistan, and Sri Lanka [12]. There was a higher incidence of adenocarcinoma of the bladder in females in Sri Lanka (4%), India (5%), and Pakistan (7%) than in the males of each of the countries (3%, 3%, and 2%, respectively). These higher rates of adenocarcinoma of the bladder in females could be due to the subtype of adenocarcinoma, clear cell adenocarcinoma, which arises from the female genital tract [13].
Smoking is a well-documented risk factor for BC and accounts for 50% of BC in males and 23% of BC in females [14]. It is interesting that, although many South Asian countries report a low prevalence of tobacco smoking, other forms of smokeless tobacco use such as inhalation (beedi) and chewing are commonly practiced and are largely underreported [15]. Therefore, the high incidence of BC in Pakistan is most likely attributed to increased tobacco use, as confirmed by the corresponding incidence of lung cancer [10] and also the high level of oral cancer, which is an indirect indicator of alternate forms of tobacco consumption [10]. Pakistan had the highest rate of female smokers (2.8%) which was reflected by the corresponding incidence of BC in females (Table 1). This incidence is likely to be compounded by increased passive-smoking rates and by the percentages of female users of smokeless tobacco (up to 47% of non-cigarette smokers) [15]. Similarly, in Sri Lanka, the cancer registry data showed that the leading sites of cancer in men in 2005 were the lip, oral cavity and pharynx, lung, and esophagus, in keeping with the relatively high prevalence of tobacco smoking and smokeless tobacco use in Sri Lankan males (24.5 [16] to 38.56% [ADIC]). Although speculative, the higher prevalence of male smokers found in the rural districts with a high proportion of late presentations of BCs in these areas may imply that the actual rates of BC in these districts could be even higher than in Colombo.
Beta-naphthylamine used by workers in the dye and rubber industries has been identified as a carcinogen for BC [17]. A large proportion of the South Asian economy is dependent on the manufacture and export of textiles and rubber products (Fig. 4); however, there was no corresponding increase in the incidence of BC.
The widespread use of pesticides is also thought to be linked to BC, but the use of these is likely similar between these countries.
The migrant South Asian population to the UK and the US had a higher incidence of BC than that in Sri Lanka, India, and Bangladesh (Table 1), with a 5- to 6-fold increase in the UK and a 2- to 5-fold increase in the US compared with Sri Lanka. However, the BC incidence rates in Pakistan were similar to or higher than the BC incidence rates in the migrant Asians in the UK and the US (Table 1).
Similarly, other cancers such as lung, breast, and prostate cancers have been shown to be higher in South Asian migrants to the UK and the US than in the South Asian population living in South Asia [9,18]. Although this could be due to better data collection, detection methods, and higher awareness in the UK and the US, this could also be a true environmental effect as shown by the Multiethnic cohort (MEC) study, in which Japanese immigrants to Hawaii initially had different incidences of cancers (such as breast and stomach) than those of the native Hawaiian population, but by the second generation, the incidences had become similar [19]. Therefore, as seen by our findings, there may also be a genetic, environmental, or dietary protection in the population living in South Asia.
A limitation of the present study is the data collection by the NCCP, especially in the war areas; thus, the incidence of BC is likely to be underrepresented. We tried to correct for this by excluding the war areas in our analysis, while emphasizing districts with better data collection facilities and by analyzing the NHSL data to confirm the incidence of BC in Sri Lanka. Additionally, the detection methods and the initial tests aiding diagnosis may differ between the countries compared. Also, the limitations of the "spot methodology" conducted by the ADIC are minimized by a bi-annual survey that is conducted in a repeatable manner in different areas.
The incidence of BC in Sri Lanka was low, in keeping with the BC rates in South Asian countries such as India. However, the rates of BC in Pakistan were much higher than in other South Asian countries, in keeping with the high rates of smoking. With comparatively high smoking rates in the rural areas of Sri Lanka along with under-reporting of BC, the incidence of BC in Sri Lanka is likely to be higher than the cancer registry data and may be similar to the reported rates of Colombo.
Remarkably, the migrant South Asian populations to the UK and the US have an increased incidence of BC compared with populations living in South Asian countries (except Pakistan), which suggests a genetic or environmental protection. Therefore, further studies analyzing the genetic and environmental protection in the South Asian population are warranted.
Figures and Tables
FIG. 1
Map of Sri Lanka denoting the districts and population estimates for 2005. Shaded districts were excluded from the study to ensure data reliability.
ACKNOWLEDGEMENTS
We would like to thank Dr. M.A.Y. Ariyaratne (Director NCCP) for granting us access to the NCCP data. We are extremely grateful to Mr. Suranga Wanniarchchi (senior project officer research and information programme, ADIC Sri Lanka) and Ms. Achala Dilrukshi (senior project officer, library and information programme, ADIC Sri Lanka) for the ADIC Sri Lanka data and Dr. Mala Tudawe of the faculty of medicine Sri Lanka for all the guidance and support. We also wish to thank Dr. Jayantha Balawardena and Mr. B.J. Ranasinghe, who were instrumental in data acquisition.
References
1. GLOBOCAN 2008: the GLOBOCAN project. International Agency for Research on Cancer [Internet]. c2010. cited 2010 Jul 1. Lyon: IARC;Available from: http://globocan.iarc.fr/.
2. Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008. (218):12–20.
3. Olfert SM, Felknor SA, Delclos GL. An updated review of the literature: risk factors for bladder cancer with focus on occupational exposures. South Med J. 2006. 99:1256–1263.
4. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J. Cancer incidence in five continents: Volume IX. 2007. Lyon: IARC.
5. WHO Statistical Information System (WHOSIS) [Internet]. c2006. cited 2010 Jan 1. Geneva: World Health Organization;Available from: http://apps.who.int/whosis/database/core/core_select_process.cfm?country=lka&indicators=nha.
6. The World Bank [Internet]. c2012. cited 2010 Nov 1. Washington: The World Bank;Available from: http://data.worldbank.org/country/sri-lanka.
7. Segi M. Cancer mortality for selected sites in 24 countries (1950-57). 1960. Sendai: Department of Public Health, Tohoku University of Medicine.
8. Ministry of Health. Cancer incidence data: Sri Lanka Year 2000. National Cancer Control Programme, Sri Lanka. 2005. Maharagama: NCCP.
9. Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008. 37:147–160.
10. Bhurgri Y, Bhurgri A, Hassan SH, Zaidi SH, Rahim A, Sankaranarayanan R, et al. Cancer incidence in Karachi, Pakistan: first results from Karachi Cancer Registry. Int J Cancer. 2000. 85:325–329.
11. Goonewardena SA, De Silva WA, De Silva MV. Bladder cancer in Sri Lanka: experience from a tertiary referral center. Int J Urol. 2004. 11:969–972.
12. Wiwanitkit V. Overview of clinical reports on urinary schistosomiasis in the tropical Asia. Pak J Med Sci. 2005. 21:499–501.
13. Adeniran AJ, Tamboli P. Clear cell adenocarcinoma of the urinary bladder: a short review. Arch Pathol Lab Med. 2009. 133:987–991.
14. Zeegers MP, Kellen E, Buntinx F, van den Brandt PA. The association between smoking, beverage consumption, diet and bladder cancer: a systematic literature review. World J Urol. 2004. 21:392–401.
15. Rafique M. Clinico-pathological features of bladder carcinoma in women in Pakistan and smokeless tobacco as a possible risk factor. World J Surg Oncol. 2005. 3:53.
16. The Tobacco Atlas [Internet]. c2012. cited 2011.06.05. New York: World Lung Foundation;Available from: http://www.tobaccoatlas.org/.
17. Case RA, Hosker ME. Tumour of the urinary bladder as an occupational disease in the rubber industry in England and Wales. Br J Prev Soc Med. 1954. 8:39–50.
18. Ranasinghe WK, Sibanda T, de Silva MV, Ranasinghe TI, Persad R. Incidence of prostate cancer in Sri Lanka using cancer registry data and comparisons with the incidence in South Asian men in England. BJU Int. 2011. 108(8 Pt 2):E184–E189.
19. Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004. 4:519–527.
20. Sri Lanka Labour Force Survey. cited November 2010. Sri lanka: The Department of Census & Statistics - Sri Lanka;Available from: http://www.statistics.gov.lk/page.asp?page=Labour%20Force.
21. Labour Statistics. cited November 2010. India: Ministry of Labour - Government of India;Available from: http://labour.nic.in/.
22. Labour Force Statistics. cited November 2010. Pakistan: Federal Bureau of Statistics (FBS) Pakistan;Available from: http://www.statpak.gov.pk/depts/index.html.
23. Winter H, Cheng KK, Cummins C, Maric R, Silcocks P, Varghese C. Cancer incidence in the south Asian population of England (1990-92). Br J Cancer. 1999. 79:645–654.
24. Altekruse SF, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review 1975-2007. cited 2010 November. Bethesda, MD: National Cancer Institute;based on November 2009 SEER data submission, posted to the SEER web site, 10. Available from: http://seer.cancer.gov/csr/1975_2007/.
25. Jain RV, Mills PK, Parikh-Patel A. Cancer incidence in the south Asian population of California, 1988-2000. J Carcinog. 2005. 4:21.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download