Abstract
Purpose
This study assessed whether 99mtechnetium dimercaptosuccinic acid (DMSA) scintigraphy used for the assessment of renal sequelae after febrile urinary tract infection (UTI) has any prognostic value for outcome measurement of vesicoureteral reflux (VUR) by retrospectively evaluating the correlation between abnormal DMSA scintigraphy results and persistence of VUR in children with febrile UTI.
Materials and Methods
The medical records of 142 children (57 boys, 85 girls) admitted with febrile UTI from January 2004 to December 2006 and who were followed up for more than 1 year were retrospectively reviewed. At the initial and follow-up visits, renal ultrasound and DMSA scans were performed within 7 days from the diagnosis and voiding cystourethrography (VCUG) was performed within 1 month in all case and follow-up evaluations.
Results
The children's mean age was 4.8±3.6 years (range, 0.3 to 14 years). The mean follow-up was 28.2±4.8 months. At the initial examination, VUR was more often associated with an abnormal DMSA scan result (83.3%) than with a normal DMSA scan result (16.7%, p=0.02). The frequency of VUR with an abnormal DMSA scan during acute UTI was significantly higher than the frequency of VUR with a normal DMSA scan (38.8% vs, 25.8%, respectively, p=0.004). Also, high-grade VUR was associated with an abnormal DMSA scan result (32.5%) more often than with a normal DMSA scan result (0%, p=0.01). Children with an abnormal DMSA scan had a lower resolution rate of VUR (17.5%) than did children with a normal DMSA scan (75.0%) at the follow-up VCUG (p=0.02).
Urinary tract infection (UTI) is one of the most common diseases in children [1], and vesicoureteral reflux (VUR) is estimated to occur in 25 to 50% of children with febrile UTI [2,3]. Approximately 30 to 40% of children with VUR have renal scarring at the time of diagnosis [3,4]. Recurrent UTI related with renal scarring and VUR are risk factors for progressive renal damage, which may lead to severe sequelae such as hypertension and permanent renal failure [5,6].
A voiding cystourethrogram (VCUG) is used to evaluate VUR. However, VCUG is an invasive procedure and has many side-effects, such as pain, irradiation, and UTI. The dimercaptosuccinic acid (DMSA) renal scan is a noninvasive test that allows for cortical imaging; this approach is currently recommended for evaluation of first-time febrile UTI because of its high sensitivity for detection of renal parenchymal injury [7]. However, it is not clearly known whether DMSA scintigraphy performed during febrile UTI has any prognostic value for outcome assessment in these children. Camacho et al. [8] advocated that children with an abnormal DMSA scan result have a higher frequency of VUR than do children with a normal DMSA scan result (48% vs. 12%) and that children with normal DMSA scan results during acute UTI have a low risk of renal damage. In the present study, we evaluated the usefulness of DMSA scans performed during febrile UTI for prediction of the severity and persistency of VUR that may lead to progressive renal damage.
A total of 142 consecutive children (57 boys and 85 girls; mean age, 6.7±2.6 years) who presented with an episode of acute febrile UTI from January 2004 to December 2006 and who were followed up for more than 1 year were included in this retrospective study. DMSA and renal ultrasound scans were performed during the first 7 days of the acute episode, and VCUG was done 1 month after the acute phase of disease to determine the presence of VUR [9]. Children with detected VUR underwent a follow-up VCUG study within 1 year after the first episode of acute UTI. VUR was graded according to the recommendations of the International Reflex Study [10] and then categorized as low grade (grade I to III) or high grade (grade IV to V). Children with bilateral reflux, voiding difficulty caused by neurogenic bladder, dysfunctional elimination syndrome, or duplicated kidney were excluded from this study. Appropriate antimicrobial prophylaxis was continued in children with a high grade of VUR and a recurrent UTI episode. For patients with breakthrough UTI and aggravated VUR, endoscopic subureteral injection of Deflux (dextranomer hyaluronic acid copolymer) was considered. This study was approved by the institutional review board in Chonnam National University Hospital.
DMSA scintigraphy was performed by using a standard protocol applied by the calculated dose of DMSA according to the body surface area performed within 7 days of diagnosis [11]. Four hours later, posterior, anterior, and each oblique view were obtained. DMSA scintigraphy was interpreted according to the recommendations of the European Association of Nuclear Medicine Pediatric Task Group [12]. A focal reduction or absence of uptake in more than one area of the kidney was considered abnormal. Relative function of the kidney of less than 45% was also classified as abnormal.
SPSS ver. 7.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Chi-square test was used to compare frequencies. Values of p<0.05 were deemed to be statistically significant.
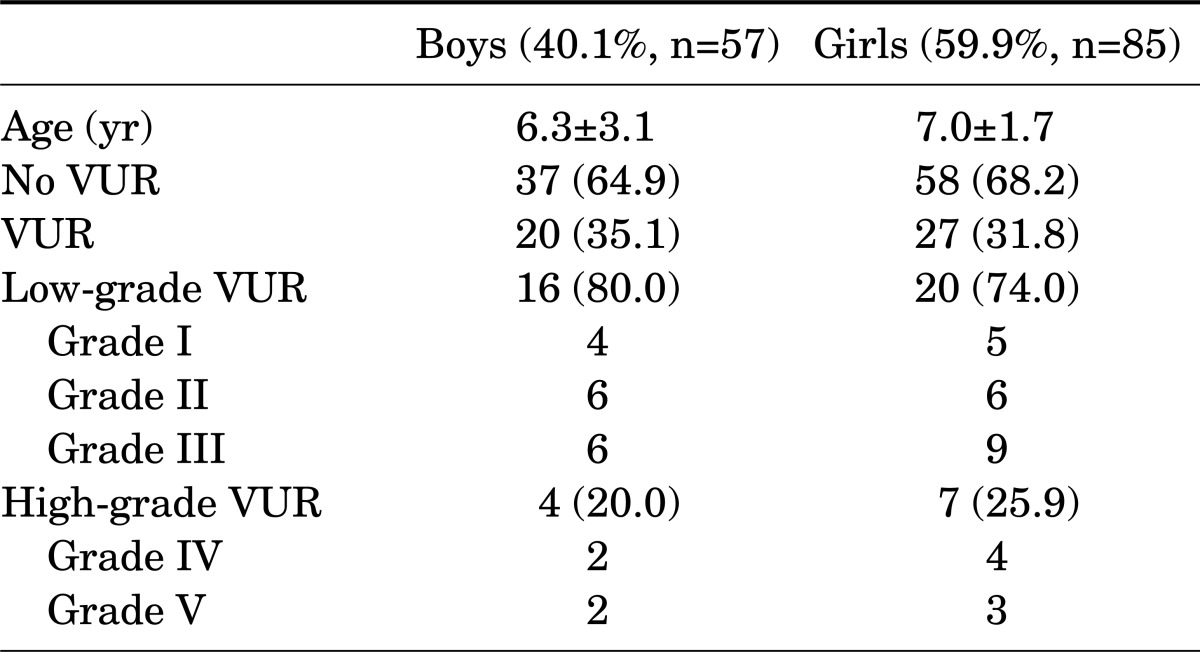
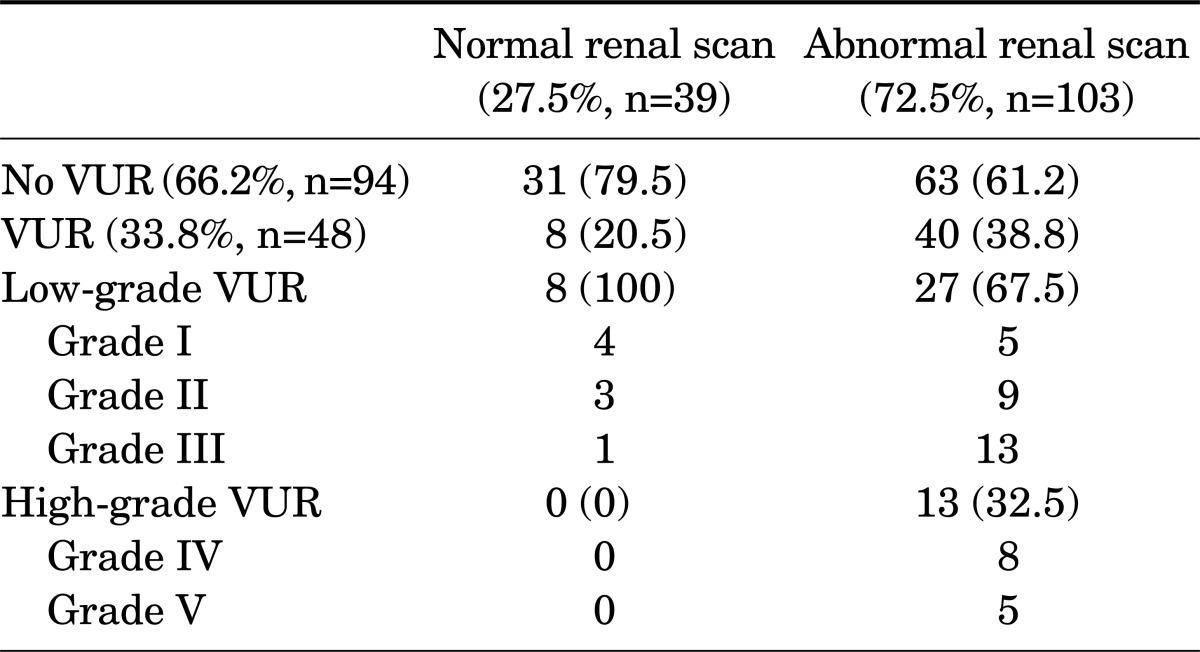
The characteristics of the patients are listed in Table 1. VUR was found in 47 children (33.1%) with febrile UTI, of whom the majority had low-grade VUR (76.6% vs. 27.1%). There were no significant differences in basal characteristics according to gender or in VUR incidence between boys and girls (p=0.2) (Table 1). At the initial examination, abnormal DMSA scintigraphy results were found in the majority (72.5%) of children with febrile UTI. The frequency of VUR with an abnormal DMSA scan result during the acute febrile UTI episode was significantly higher than that of VUR with a normal DMSA scan result (38.8% vs. 25.8%, p=0.004). In all children with a normal renal scan result, the reflux grade was low-grade; high-grade VUR was not found in this group (Table 2). None of the 13 patients with high-grade VUR had a normal DMSA scan result.
At the follow-up evaluation, the rate of VUR resolution in children with an abnormal DMSA scan result during the acute febrile UTI episode (7/40) was significantly lower than the rate in children with a normal DMSA scan result (6/8; 17.5% vs. 75.0%, respectively, p<0.05) (Table 3). Most of the children with VUR with an abnormal DMSA scan result at the initial visit had persistent VUR (33/40, or 82.5%) at the follow-up evaluation.
In the present study, VUR was found in 33.8% of children with febrile UTI, and abnormal DMSA scintigraphy results were found in 72.5% of children with febrile UTI. The purpose of this study was to assess the usefulness of DMSA study performed during acute febrile UTI in identifying children at risk of VUR or of resolution of VUR. In the present study, VUR had a high rate of association with abnormal DMSA scan results compared with normal DMSA scan results. Furthermore, the frequency of VUR with an abnormal DMSA scan result during acute UTI was significantly higher than that of VUR with a normal DMSA scan result. The present findings are consistent with the view that the DMSA scan obtained in children with febrile UTI is useful in the formulation of a proper management plan for those children in the clinical setting. Those with abnormal DMSA results and renal scarring are more likely to display VUR and are at increased risk of persistent VUR. This information is useful in counseling children and parents about the natural course of VUR and in prediction of VUR resolution during the follow-up period.
VCUG is an unpleasant and invasive procedure that involves radiation exposure and introduces the risk of catheter-induced UTI [9]. These risks can dissuade clinicians from routinely performing VCUG in children after their first febrile UTI. Instead of VCUG, DMSA scans can be used to predict VUR. DMSA scintigraphy has high sensitivity for detection of renal abnormalities and is considered the gold standard for diagnosis of renal damage [13]. In the present study, 103 of 142 children (72.5%) presented with abnormal findings on the DMSA scintigraphy performed during the acute period of UTI. Preda et al. [9] reported that DMSA abnormalities are found in 51% of children with febrile UTI when DMSA is performed early in the course of UTI. The relatively higher incidence of abnormal DMSA studies found in our study may be attributed to our including recurrent UTI in our data.
VUR is one of the most frequent risk factors for the development of renal damage. In the present study, VUR was found in 33.8% of the children with acute febrile UTI, whereas other studies have reported an incidence of VUR of 24 to 25% of children with febrile UTI [14,15]. An abnormality in a DMSA scan could occur in the absence of VUR, and in the present study, as many as 63 of 103 children with an abnormal DMSA scan result showed no reflux. This finding suggests that more than reflux itself is involved in the pathogenesis of parenchymal defects and renal scarring; additional factors could include bacterial virulence factors or host immunity [16].
In an earlier retrospective study, Temiz et al. [17] showed that a strategy to perform VCUG only in patients with abnormalities on the DMSA scan to identify patients with high-grade VUR would have reduced the number of VCUG procedures by half. A recent study by Tseng et al. [18] retrospectively analyzed the medical records of 142 children with VUR and reported that all the children with grades III to V VUR (21 patients) had an abnormal DMSA scan result. Those authors further stated that if VCUG had been performed only in children with an abnormal scan result, 41 of 142 (29%) investigations could have been omitted. In the present study, the incidence of VUR with a normal DMSA scan result was very low (8/39, or 20.5%), and the degree of VUR in those children was low-grade (grade I, n=4; grade II, n=3; grade III, n=1) and clinically insignificant. These results support the concept of not performing VCUG routinely in children with UTI.
Many studies have reported a spontaneous VUR resolution rate of 53 to 68% during follow-up, with the rate differing according to the grade of VUR [19,20]. A recent study reported that low-grade VUR (grades I to III) diagnosed after UTI resolves significantly more rapidly than does high-grade VUR (grades IV to V) [21]. Camacho et al. [8] evaluated the prognostic value of DMSA renal scans performed during febrile UTI and reported that the frequency of VUR was higher by as much as 48% when the DMSA scan result was abnormal than the frequency of VUR when the DMSA scan result was normal in children (12%). Another study reported that the rates of 2-year reflux resolution in abnormal and normal renal scan groups were 13% and 53%, respectively [22]. In our study, all the subjects with high-grade reflux showed an abnormal renal scan, and the VUR of those children did not show spontaneous resolution on follow-up evaluation. The rate of VUR resolution differed depending on the DMSA scan result during the acute UTI. At the follow-up evaluation, the rate of VUR resolution in children with an abnormal DMSA scan result during the acute febrile UTI was significantly lower than in children with a normal DMSA scan result. Most of the children with VUR with an abnormal DMSA scan result at the initial visit were found to have persistent VUR at the follow-up evaluation. We suggest that in children with an abnormal result on a DMSA renal scan during acute febrile UTI, most of the reflux does not resolve after follow-up evaluation, in contrast with the case in children with a normal result on a DMSA renal scan, in whom reflux tends to resolve. Furthermore, in the present study, in children with a normal renal scan, most of the reflux was low-grade, whereas none of the patients with high-grade VUR had a normal DMSA scan result. These results concur with previous reports that higher grades of reflux are associated with an increased incidence of an abnormal result on a DMSA renal scan [23,24] and a strong association between reflux grade and the incidence of renal scarring. However, Goldman et al. [25] reported that renal scarring was not related in children with pyelonephritis and low-grade reflux and that there was no correlation between parenchymal defects and recurrent UTI.
There are several limitations to our study. Concerning the reversible defect of renal damage, it is important to consider the timing of DMSA renal scintigraphy. We made every effort to conduct the DMSA scintigraphy within the acute period of febrile UTI within 7 days of diagnosis. However, variations in this timing did occur in a small number of cases. VCUG was performed within 1 month after the diagnosis, but with small variation among the subjects. These variable factors between the subjects included in this study should be considered. We excluded children with bilateral VUR and could not evaluate the DMSA renal scan results according to the frequency of febrile UTI or differences in gender or age. Another limitation of our study is that we did not distinguish between first UTI and recurrent UTI. In our study, the mean age of the children with febrile UTI was relatively older than in other similar studies. Renal scars may have developed in older children after previously unrecognized UTIs, and these scars cannot be differentiated from acute photon defects on DMSA renal scintigraphy. To avoid such a limitation, the current study limited the age to younger than 1 year [26].
Abnormal DMSA scan results during febrile UTI were associated with high-grade and persistent VUR. DMSA scanning performed during febrile UTI was useful as an indicator of reflux resolution in childhood. A DMSA scan should be considered when counseling families about the prognosis of febrile UTI with respect to VUR and renal damage.
References
1. Rushton HG. Urinary tract infections in children. Epidemiology, evaluation, and management. Pediatr Clin North Am. 1997; 44:1133–1169. PMID: 9326956.
2. Gleeson FV, Gordon I. Imaging in urinary tract infection. Arch Dis Child. 1991; 66:1282–1283. PMID: 1661570.

3. Smellie JM, Ransley PG, Normand IC, Prescod N, Edwards D. Development of new renal scars: a collaborative study. Br Med J (Clin Res Ed). 1985; 290:1957–1960.

4. Bellinger MF, Duckett JW. Vesicoureteral reflux: a comparison of non-surgical and surgical management. Contrib Nephrol. 1984; 39:81–93. PMID: 6744882.
5. Merrick MV, Notghi A, Chalmers N, Wilkinson AG, Uttley WS. Long-term follow up to determine the prognostic value of imaging after urinary tract infections. Part 1: Reflux. Arch Dis Child. 1995; 72:388–392. PMID: 7618902.

6. Merrick MV, Notghi A, Chalmers N, Wilkinson AG, Uttley WS. Long-term follow up to determine the prognostic value of imaging after urinary tract infections. Part 2: Scarring. Arch Dis Child. 1995; 72:393–396. PMID: 7618903.

7. Lee JH, Kim MK, Park SE. Is a routine voiding cystourethrogram necessary in children after the first febrile urinary tract infection? Acta Paediatr. 2012; 101:e105–e109. PMID: 22040289.

8. Camacho V, Estorch M, Fraga G, Mena E, Fuertes J, Hernandez MA, et al. DMSA study performed during febrile urinary tract infection: a predictor of patient outcome? Eur J Nucl Med Mol Imaging. 2004; 31:862–866. PMID: 14758509.

9. Preda I, Jodal U, Sixt R, Stokland E, Hansson S. Normal dimercaptosuccinic acid scintigraphy makes voiding cystourethrography unnecessary after urinary tract infection. J Pediatr. 2007; 151:581–584. 584.e1PMID: 18035134.

10. Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Möbius TE. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol. 1985; 15:105–109. PMID: 3975102.
11. Gordon I, Piepsz A, Sixt R. Auspices of Paediatric Committee of European Association of Nuclear Medicine. Guidelines for standard and diuretic renogram in children. Eur J Nucl Med Mol Imaging. 2011; 38:1175–1188. PMID: 21503762.

12. Piepsz A, Hahn K, Roca I, Ciofetta G, Toth G, Gordon I, et al. Paediatric Task Group European Association Nuclear Medicine. A radiopharmaceuticals schedule for imaging in paediatrics. Eur J Nucl Med. 1990; 17:127–129. PMID: 2279492.

13. Majd M, Nussbaum Blask AR, Markle BM, Shalaby-Rana E, Pohl HG, Park JS, et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology. 2001; 218:101–108. PMID: 11152787.
14. Jakobsson B, Nolstedt L, Svensson L, Soderlundh S, Berg U. 99mTechnetium-dimercaptosuccinic acid scan in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol. 1992; 6:328–334. PMID: 1343562.

15. Ditchfield MR, de Campo JF, Nolan TM, Cook DJ, Grimwood K, Powell HR, et al. Risk factors in the development of early renal cortical defects in children with urinary tract infection. AJR Am J Roentgenol. 1994; 162:1393–1397. PMID: 8192006.

16. Lomberg H, Hanson LA, Jacobsson B, Jodal U, Leffler H, Eden CS. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983; 308:1189–1192. PMID: 6341837.

17. Temiz Y, Tarcan T, Onol FF, Alpay H, Simşek F. The efficacy of Tc99m dimercaptosuccinic acid (Tc-DMSA) scintigraphy and ultrasonography in detecting renal scars in children with primary vesicoureteral reflux (VUR). Int Urol Nephrol. 2006; 38:149–152. PMID: 16502071.

18. Tseng MH, Lin WJ, Lo WT, Wang SR, Chu ML, Wang CC. Does a normal DMSA obviate the performance of voiding cystourethrography in evaluation of young children after their first urinary tract infection? J Pediatr. 2007; 150:96–99. PMID: 17188624.

19. Sillen U. Vesicoureteral reflux in infants. Pediatr Nephrol. 1999; 13:355–361. PMID: 10454790.
20. Wennerstrom M, Hansson S, Jodal U, Stokland E. Disappearance of vesicoureteral reflux in children. Arch Pediatr Adolesc Med. 1998; 152:879–883. PMID: 9743033.

21. Schwab CW Jr, Wu HY, Selman H, Smith GH, Snyder HM 3rd, Canning DA. Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol. 2002; 168:2594–2599. PMID: 12441993.

22. Nepple KG, Knudson MJ, Austin JC, Cooper CS. Abnormal renal scans and decreased early resolution of low grade vesicoureteral reflux. J Urol. 2008; 180(4 Suppl):1643–1647. PMID: 18715588.

23. Merguerian PA, Jamal MA, Agarwal SK, McLorie GA, Bagli DJ, Shuckett B, et al. Utility of SPECT DMSA renal scanning in the evaluation of children with primary vesicoureteral reflux. Urology. 1999; 53:1024–1028. PMID: 10223500.

24. Stokland E, Hellstrom M, Jacobsson B, Jodal U, Sixt R. Renal damage one year after first urinary tract infection: role of dimercaptosuccinic acid scintigraphy. J Pediatr. 1996; 129:815–820. PMID: 8969722.

25. Goldman M, Bistritzer T, Horne T, Zoareft I, Aladjem M. The etiology of renal scars in infants with pyelonephritis and vesicoureteral reflux. Pediatr Nephrol. 2000; 14:385–388. PMID: 10805465.

26. Lee YJ, Lee JH, Park YS. Risk factors for renal scar formation in infants with first episode of acute pyelonephritis: a prospective clinical study. J Urol. 2012; 187:1032–1036. PMID: 22264451.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download