Abstract
Purpose
The metabolic syndrome (MS) has been accepted as an important cause of benign prostatic hyperplasia (BPH) in old age. However, there are no studies of the influence of MS on prostate volume in relatively young adults. We evaluated the relationship between parameters of MS and prostate volume in men under 50 years of age who visited our health promotion center.
Materials and Methods
A total of 968 men aged 30 to 49 years were enrolled from March 2009 to June 2010. Prostate volume by transrectal ultrasonography of the prostate, serum prostate-specific antigen, digital rectal examination, urinalysis, and MS-related parameters were investigated. We evaluated the correlations of prostate volume with MS and MS-related parameters.
Results
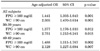
Prostate volume was not significantly larger in the MS group (18.4 cc; range: 14.3-23.1 cc) than in the non-MS group (17.8 cc; range, 13.6-21.6 cc). The prostate volumes in subjects with abnormal fasting plasma glucose (FPG) (18.9 cc; range, 14.3-22.7 cc) and abnormal waist circumference (WC) (19.5 cc; range, 15.6-23.7 cc) were significantly larger than those of subjects with normal parameters (16.9 [range, 12.7-20.4] cc and 17.5 [range, 13.3-21.2] cc, respectively; p=0.001). The logistic regression analysis showed the FPG level and WC to have a significantly positive correlation with the prostate volume (odds ratios: 1.441 [95% CI: 1.303-1.643] and 2.305 [95% CI: 1.470-3.614], respectively).
As the common benign tumor in male, benign prostatic hyperplasia (BPH) develops as a age-related phenomenon in almost all men, starting at approximately 40 years of age. Clinically, BPH leads to a progressive enlargement of the prostate gland, causing both obstructive and irritative lower urinary tract symptoms (LUTS). The lifetime risk for a 50-year-old man to undergo a prostatectomy for BPH has been estimated to be as high as 40% [1].
BPH is a specific histopathologic entity characterized by stromal and epithelial cell hyperplasia. For over a century, there have been two known etiologic factors for the pathogenesis of BPH: aging and androgens [2]. Moreover, family history, ethnicity, smoking, type II diabetes mellitus (DM), high-density lipoprotein cholesterol (HDL-C), high insulin content, high blood pressure, and obesity have been reported to be risk factors [3].
Many reports have discussed the correlations of metabolic syndrome (MS) and prostate volume. However, despite such existing epidemiological and pathophysiologic evidence, there are many disputes in considering MS as a risk factor for BPH. One of the reasons for this dispute is the paucity of large epidemiological studies. Another reason is that most studies that analyzed the relationship between MS and BPH were conducted in older men more than 50 years of age.
Therefore, unlike the previous studies that reviewed BPH and MS in old age groups, in the present study, we analyzed its associations with MS and components of MS by investigating the prostate volume of relatively healthy, young male subjects (in their fourth to fifth decades) without underlying disease.
Among 75,368 men older than 30 and younger than 50 years of age who came to the Health Promotion Center at the Kangbuk Samsung Hospital for a routine health check-up between March 2009 and June 2010, data were retrospectively obtained from 986 men who underwent transrectal ultrasonography (TRUS) of the prostate. TRUS was performed in subjects who came to the Health Promotion Center as part of a basic examination included in a health screening program or as a test selected by the subjects themselves. The health screening program available at our Health Promotion Center includes anthropometric measurements (height, weight, and waist circumference [WC]), blood test (a complete blood cell count, basic chemistry, serologic test, blood coagulation test, thyroid function test, and assay for tumor markers), stool/urine analysis, abdominal ultrasonography, gastrofiberscopy, chest radiography, pulmonary function test, electrocardiography, and detailed clinical examination. All subjects were also asked to complete a questionnaire designed to assess sociodemographic factors, comorbidities, and current or past medications. The Institutional Review Board of the Kangbuk Samsung Hospital approved all the procedures involved in sampling and data collection, and written informed consent was obtained from all participants. Among the 986 men, 138 men were excluded from the study for reasons including taking medications for BPH (n=47), having a history of type I insulin-dependent DM (n=22), having abnormal serum prostate-specific antigen (PSA) (≥4 ng/ml; n=11), or having a suspicion of prostate cancer by TRUS or digital rectal examination (DRE; n=1). In addition, 57 men showing pyuria on urinalysis were excluded from the study.
Metabolic syndrome was defined by using the criteria established by the National Cholesterol Education Program-Adult Treatment Panel III-American Heart Association/National Heart, Lung, and Blood Institute (NCEP-ATPIII-AHA/NHLBI) statement, published in 2005 [4]. Central obesity was defined as WC ≥90 cm in men or ≥80 cm in women by the modified ATP III guideline that the WHO-Western Pacific Region (WPR) and the International Association for the Study of Obesity (IASO) presented for Asian populations in 2000. MS was diagnosed when at least three of the following criteria were present: WC of ≥90 cm, triglyceride (TG) levels of ≥150 mg/dl or undergoing treatment for hypertriglyceridemia, HDL-C levels of <40 mg/dl or undergoing treatment for low HDL-C, blood pressure (BP) of ≥130/85 mm Hg or undergoing treatment for hypertension, and fasting plasma glucose (FPG) of ≥100 mg/dl or undergoing treatment for hyperglycemia.
All parameters were measured on fresh serum obtained after the subjects had fasted for 12 hours overnight. Serum total cholesterol, TG, HDL-C, and FPG were measured by enzymatic methods. Serum PSA values were measured by using a Tandem-R PSA immunoradiometric assay. The prostate volume was calculated according to the prostate ellipsoid formula, multiplying the largest anteroposterior (height, H), transverse (width, W), and cephalocaudal (length, L) prostate diameters by 0.524 (HxWxLx π/6) by using TRUS (ALOKA®, prosound-α5sv).
All analyses were performed by using the PASW ver. 17.0 (SPSS Inc., Chicago, IL, USA). Associations were assessed between each of the metabolic components, as well as MS, and prostate volume. Prostate volumes were compared in men with and without MS, and with and without each metabolic component, by using the Mann-Whitney U-test. A p value of less than 0.05 was deemed as significant. Continuous variables were expressed as the mean±standard deviation (SD), and because most variables in this report were not normally distributed, we preferred to use nonparametric statistics, namely, the median value. A multiple logistic regression model was performed to examine the association between MS and prostate volume. We used the risk ratio with 95% confidence intervals (CIs) to evaluate the association between significant variables and prostate volume. Adjusted odds ratios (ORs) with accompanying 95% CIs are reported.
The mean age of the entire subject population was 41.4±5.2 years. The mean prostate volume in all subjects was 18.4±6.3 cc. The mean value of each component of MS and PSA are shown in Table 1.
The prostate volume was not significantly larger in the MS group. The median prostate volumes in the MS and non-MS groups were 18.4 cc (range, 14.3-23.1 cc) and 17.8 cc (range, 13.6-21.6 cc), respectively. There was no statistically significant difference between the two groups (p>0.05). In addition, there were no statistically significant differences in the prostate volume according to the presence of MS in the two age subgroups (30-39 and 40-49 years; Table 2).
Men with abdominal obesity (WC≥90 cm) and men with abnormal FPG levels (≥100 mg/dl) had a larger prostate volume than did the normal control group. The median prostate volumes in the abdominal obesity group and the normal WC group were 19.5 cc (range, 15.6-23.7 cc) and 17.5 cc (range, 13.3-21.2 cc), respectively (p<0.05). The prostate volumes according to normal and abnormal FPG level were 16.9 cc (range, 12.7-20.4 cc) and 18.9 cc (range, 14.3-22.7 cc), respectively (p<0.05). When the prostate volume was further analyzed according to age subgroups, the prostate volumes in men with abdominal obesity or abnormal FPG levels were also significantly larger than in the normal control groups (p<0.05) (Table 2).
The results of the multivariate analysis after adjustment for age and each MS factor showed that abdominal obesity (OR=2.305; 95% CI: 1.470-3.614, p=0.001) and an abnormal FPG level (OR=1.441; 95% CI: 1.303-1.643, p=0.001) were strongly associated with enlarged prostate volume (≥20 cc) (Table 3).
In the United States, the prevalence rate of MS has been reported variously ranging from 2.4% to 43.5%. According to data reported in United States in 2005, the prevalence rate of MS according to the NCEP-ATP III criteria reached 34.6%, and a prevalence rate of 28% was reported when the same criteria were applied in Korea [5].
MS is a constellation of multiple metabolic and cardiovascular disease risk factors, at the center of which is insulin resistance (a state in which muscle, liver, and fat tissues have reduced sensitivity to insulin) and compensatory hyperinsulinemia. Investigators have reported a strong influence of age on the presence of MS, which affects 43.5% of those aged 60 to 69 years [6]. Similarly, BPH is seen frequently in males older than 50 years. About 60% of men aged over 50 years have histological evidence of BPH and, after age 70, the proportion increases to 80% [7]. Historically, the development of BPH focused on the interplay between genetic and hormonal factors. More recently, however, a growing body of evidence has demonstrated a strong and independent link between BPH and obesity and MS. This relationship has implications for the pathophysiology and treatment of these disorders. Furthermore, this association implies that modifiable risk factors may play a role in the pathogenesis of these entities [8].
Despite the high prevalence of BPH in aged men, our understanding of the disease pathogenesis is far from complete. Although the etiology of BPH is not well understood, several theories have been proposed to explain the pathogenesis of BPH [9]. Testosterone-dihydrotestosterone (DHT) signaling and mesenchymal-epithelial interactions are required for normal prostaticgrowth and are known to play an important role in the progression of disease [10]. Many risk factors for BPH, such as insulin, insulin-like growth factors (IGFs), and dyslipidemia, might act through androgen-independent mechanisms [11,12].
The underlying cause of MS also continues to challenge experts, but both insulin resistance and central obesity are considered to be significant factors [13]. Recent studies suggest that hyperinsulinemia secondary to insulin resistance and the components of MS are risk factors for the development of BPH [14-16]. Furthermore, MS may play a role in BPH pathogenesis. Several experimental and clinical reports indicate the critical role of obesity and insulin-resistance-associated complications in the pathogenesis of BPH [3,15,17,18]. The increased incidence of BPH in insulin-resistant and diabetic populations strengthens the relationship between these two pathological conditions and makes this an increasingly relevant problem [19]. Insulin resistance is a condition in which a normal level of insulin elicits a subnormal response. It is a condition that is associated with a group of disorders such as obesity, dyslipidemia, elevated fasting glucose level, hyperinsulinemia, and hypertension. In addition to type 2 DM and cardiovascular disease, patients with insulin resistance syndrome are at higher risk for BPH [20]. Some isolated reports oppose the view regarding the relationship between insulin resistance and BPH [21,22].
The insulin-resistance-associated disorders such as obesity, dyslipidemia, hypertension, sympathetic overactivity, and hyperinsulinemia have been implicated in the pathogenesis of BPH. The prevalence of obesity is increasing all over the world due to sedentary lifestyles. Obesity has been implicated in the etiology of BPH because of its influence on metabolic and endocrine changes. Recent findings indicate that obesity substantially increases the risk for BPH. In the study conducted by Dahle et al, an increased serum insulin level was related to increased BPH risk, and Parson et al reported the correlations of prostate volume with body mass index, elevated fasting blood sugar level, and DM [16,17]. The Health Professionals Follow-up Study, a prostate cancer prevention trial, and a case-control study of Italian men showed a positive association between obesity and BPH [16]. The fasting serum level of insulin in the patients with higher waist-to-hip ratio was significantly high, indicating the involvement of insulin in the progression of disease [16]. Several independent studies have further supported the association between obesity and hyperinsulinemia [23,24]. In a population-based study conducted in Korea, Jang et al reported that there was a significant correlation between each MS factor and prostate volume [25]. Koo et al also reported that MS is associated with prostate volume-related factors, but not to voiding dysfunction in Korean men 60 years of age or older. Among the subcategories of MS, they reported that obesity is the factor most strongly related to prostate volume [26]. Few reports argue that obesity is associated with increased estrogen-to-androgen ratio and sympathetic activity, both of which are individually hypothesized to promote the development of prostatic hyperplasia [14].
Obesity can augment prostatic growth either by promoting the development of insulin resistance and secondary hyperinsulinemia or by increasing the estrogen-to-androgen ratio. One more pathway that explains the increased risk for BPH in hyperinsulinemia is the IGF axis. IGF-1 is a strong mitogen and increases cell proliferation and induces apoptosis in many tissues, including prostatic stroma and epithelium [27].
In this study, we found that obesity and DM were risk factors for BPH in young men, but the presence of MS was not. According to the study of Gupta et al, MS had no contributing effect on BPH in a prospective study of the causal relationship of MS and BPH through long-term follow-up observation (mean of 15.6 years) [21]. The results should be interpreted with caution, however. Several studies have postulated that MS and BPH may be related [3,28]. Our report is the only study to assess whether relatively young men with MS had an increased risk of BPH, and we found no relationship between the two. Apart from abnormal FPG and obesity, none of the other markers of the MS were associated with an increased risk of BPH. The results from other prospective studies have not shown a consistent relationship between any of the MS markers and BPH [14,29]. Thus, considering the evidence from the longitudinal studies, it is unlikely that a causative association exists between the MS and BPH.
We did not find any relationship between prostate volume and HDL-C or TG. In the study conducted by Rohrmann et al using data from NHANES III, they also did not find any relationship between LUTS and HDL-C, low density lipoprotein, TG, or total cholesterol. Overall, dyslipidemia is likely to have a limited role, if any, in the etiology of BPH.
We also did not find any relationship between prostate volume and BP. In contrast, Gann et al, in a smaller prospective study involving 640 men, found an increased odds of BPH with a higher diastolic BP and no effect with systolic BP. The mechanism of the association between BP and BPH/LUTS remains unknown, but a role of sympathetic tone has been hypothesized [30].
Unlike the previous studies, the present study analyzed the substantial correlations of BPH with MS in relative younger men aged 30 to 49 years. The present data show the possibility of young adults with obesity or DM having a larger prostate volume, which is similar to the clinical observation that men suffering from DM and obesity have a larger prostate gland volume than do men without these conditions at old ages. According to the study of Berry et al, the prostate volume doubling time was 4.5 years among BPH patients aged 30 to 50 years, but the reported the doubling times of the patients aged 50-71 years was 10 years and that of patients aged more than 70 years was 100 years. Also, they also reported that a fast growth speed of prostate volume in younger men with BPH [7]. This study suggests that it is possible that the elements of MS may have different effects on the younger and the older. Also, if young men have obesity and DM, they are predisposed to early and severe development of BPH owing to the effect of obesity and DM on the naturally rapidly growing prostate compared with old men. Therefore, more caution is needed when young men have obesity and DM for early diagnosis and treatment of BPH. Also, modification of lifestyles and high-fat diets in young men with obesity and DM are needed to prevent the development of BPH.
In our study, the analyses were cross-sectional, and thus causal and longitudinal relations were not addressed. There was the potential for selection bias because the subjects were the populations who visited the health promotion center for routine medical examination and not for the treatment or diagnosis of LUTS/BPH. Also, we did not consider the subject's lifestyle patterns of diet habits, exercise, and hormonal status including testosterone. Despite the aforementioned limitations, however, this study is valuable in that it proposed the possibility of early or severe BPH development in young men with obesity or DM.
In young men aged 30 to 49 years old, the presence of MS was not correlated with prostate volume. However, men with abnormal FPG and WC had larger prostates than did normal groups. The risk of prostate enlargement increased with increasing FPG and WC. Obesity and DM could be more important factors than MS in prostate volume enlargement in relatively young adults.
Figures and Tables
References
1. Oesterling JE. Benign prostatic hyperplasia: a review of its histogenesis and natural history. Prostate Suppl. 1996. 6:67–73.
2. Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995. 22:237–246.
3. Hammarsten J, Högstedt B, Holthuis N, Mellström D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998. 1:157–162.
4. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005. 112:2735–2752.
5. Lim S, Park KS, Lee HK, Cho SI. Changes in the characteristics of metabolic syndrome in Korea over the period 1998-2001 as determined by Korean National Health and Nutrition Examination Surveys. Diabetes Care. 2005. 28:1810–1812.
6. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002. 287:356–359.
7. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984. 132:474–479.
8. Moul S, McVary KT. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr Opin Urol. 2010. 20:7–12.
9. Bosch RJ. Pathogenesis of benign prostatic hyperplasia. Eur Urol. 1991. 20:Suppl 1. 27–30.
10. Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003. 253:165–174.
11. Ikeda K, Wada Y, Foster HE Jr, Wang Z, Weiss RM, Latifpour J. Experimental diabetes-induced regression of the rat prostate is associated with an increased expression of transforming growth factor-beta. J Urol. 2000. 164:180–185.
12. Barnard RJ, Aronson WJ. Benign prostatic hyperplasia: does lifestyle play a role? Phys Sportsmed. 2009. 37:141–146.
13. Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004. 53:2087–2094.
14. Giovannucci E, Rimm EB, Chute CG, Kawachi I, Colditz GA, Stampfer MJ, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994. 140:989–1002.
15. Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001. 39:151–158.
16. Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002. 168:599–604.
17. Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006. 91:2562–2568.
18. Hammarsten J, Damber JE, Karlsson M, Knutson T, Ljunggren O, Ohlsson C, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009. 12:160–165.
19. Sarma AV, Kellogg Parsons J. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol Rep. 2009. 10:267–275.
20. Kasturi S, Russell S, McVary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006. 7:288–292.
21. Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology. 2006. 68:1198–1205.
22. Park HK, Lee HW, Lee KS, Byun SS, Jeong SJ, Hong SK, et al. Relationship between lower urinary tract symptoms and metabolic syndrome in a community-based elderly population. Urology. 2008. 72:556–560.
23. Kogai MA, Lutov UV, Selyatitskaya VG. Hormonal and biochemical parameters of metabolic syndrome in male patients with body weight excess and obesity. Bull Exp Biol Med. 2008. 146:806–808.
24. Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009. 115:86–96.
25. Jang TH, Son JH, Kim JI, Jang SH. Metabolic syndrome and benign prostatic hyperplasia: a study focused on the correlation between metabolic syndrome factors and prostate volume and prostate-specific antigen. Korean J Urol. 2008. 49:986–991.
26. Koo KC, Cho KS, Kang EM, Kwon SW, Hong SJ. The relationship between metabolic syndrome and prostate volume in men over sixties who underwent prostate health check-up. Korean J Urol. 2008. 49:813–817.
27. Peehl DM, Cohen P, Rosenfeld RG. The role of insulin-like growth factors in prostate biology. J Androl. 1996. 17:2–4.
28. Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond). 2005. 29:310–316.
29. Burke JP, Rhodes T, Jacobson DJ, McGree ME, Roberts RO, Girman CJ, et al. Association of anthropometric measures with the presence and progression of benign prostatic hyperplasia. Am J Epidemiol. 2006. 164:41–46.
30. Gann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995. 26:40–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download