Abstract
Purpose
We aimed to investigate the detection of nanobacteria (NB) from expressed prostatic secretions (EPS) in patients with category III chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) and from vaginal swabs in patients with vaginitis by reverse transcriptase polymerase chain reaction (RT-PCR) and to evaluate the association between NB and Neisseria gonorrhea, Chlamydia trachomatis, Ureaplasma urealyticum (U. urealyticum), Mycoplasma hominis, Trichomonas vaginalis, and Mycoplasma genitalium.
Materials and Methods
A group of 11 men attending a specialized CP/CPPS clinic and a group of 157 women who reported symptoms of lower genital tract infection were enrolled in this study. NB were detected by RT-PCR. A Seeplex Sexually Transmitted Disease Detection assay (Seegene Inc., Seoul, Korea) was used that could detect DNA for 6 types of sexually transmitted pathogens.
Results
In EPS samples, the detection rate of NB in patients with CP/CPPS was 9.1%, and 9 (5.7%) of 157 vaginitis patients showed positive results in RT-PCR for NB in vaginal swabs. Associations observed among the 7 microorganisms included 6 (54.5%) patients who tested positive on EPS and 75 (47.8%) patients who tested positive on vaginal swabs. Five patients with vaginitis were found to have monoinfection of NB (6.7%).
Conclusions
We found that conventional RT-PCR for NB was rapid, simple, low in cost, and easily available for the detection of NB, and that NB may be a possible etiological factor for vaginitis and CP/CPPS. The prevalence of U. urealyticum among the four patients with NB coinfection was 75%; the presence of U. urealyticum might therefore raise suspicion for nanobacterial infection.
Category III chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is the category with the highest incidence, accounting for 60% to 90% of those with prostatitis [1]. Bacterial pathogens have not been found in prostatic tissue, urine, or prostatic fluid by conventional culture; however, prostatic inflammation and inflammatory markers are often identified [2]. This suggests the existence of yet unknown infectious pathogens and the importance of identifying these pathogens for the diagnosis and treatment of type III prostatitis.
The female genital tract represents a highly dynamic environment, with a resident microflora consisting of a variety of different species. The coexistence of different sexually transmitted microorganisms is very common and is due to several factors, including a common route of transmission, the sexual behavior of the host, and the resident flora [3]. Infectious vaginitis accounts for 90% of all cases in women of reproductive age and is represented by the triad of candidiasis, bacterial vaginosis, and trichomoniasis. Other less common infections are caused by gonorrhea, chlamydia, mycoplasma, herpes, campylobacter, and some parasites. Vaginal infections are often (varies between 20% and 40% of vaginal infections) a mix of various etiologies, which presents challenges for treatment. Indeed, when only one cause is treated, the other pathogens can gain resistance and induce relapses and recurrences. Therefore, the key factor is to obtain a precise diagnosis and to provide treatment with a broad-spectrum anti-infective.
Nanobacteria (NB) are newly discovered infectious agents of 100-500 nm in size with a 16S ribosomal RNA (rRNA) gene sequence and slow growth and a doubling time of about 3 days [4]. They are fastidious and difficult to culture but can be detected with standard microbiological methods [5-7]. In vivo, NB are found to be voided mainly through the urinary system, and they have been isolated within the genitourinary tract, including in polycystic disease, renal calculi, and chronic prostatitis [2]. Furthermore, nanobacterial infection of malignant ovarian tissue contributes to mechanisms leading to the formation of calcified deposits known as psammoma bodies [8].
In this study, we aimed to investigate the detection of NB from expressed prostatic secretions (EPS) in patients with CP/CPPS and from vaginal swabs in patients with vaginitis by reverse transcriptase polymerase chain reaction (RT-PCR) and to evaluate the association between NB and Neisseria gonorrhea (N. gonorrhea), Chlamydia trachomatis (C. trachomatis), Ureaplasma urealyticum (U. urealyticum), Mycoplasma hominis (M. hominis), Trichomonas vaginalis (T. vaginalis), and Mycoplasma genitalium (M. genitalium).
A group of 11 men attending a specialized CP/CPPS clinic of the Urology Department of the hospital (mean age, 43.5 years) were enrolled in the study. Prostatic fluid samples were collected from outpatients with refractory type IIIB prostatitis by aseptic technique by use of the Mearses-Stamey four glass urine collection method. All patients must have abstained from sexual activity for at least 4 days before sample collection. The urethral orifice was disinfected with benzalkonium chloride. Symptoms were quantified by the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) [9]. All patients had a complete history, physical examination, wet mount examination, urine culture, and EPS. Because the routine culture results of the first voided bladder specimen, second midstream bladder specimen, EPS, and urine sample after prostatic massage were negative, we could exclude cystitis and urethritis. The control group included 5 healthy men (mean age, 40.9 years) without symptoms. A group of 157 women of reproductive age attending the Obstetrics and Gynecology Department of the same hospital (mean age, 38 years) were enrolled in this study. All women reported symptoms of lower genital tract infection (vaginal discharge or vulvar or vaginal complaints). Three cotton swabs were obtained from the posterior vaginal fornix of each patient. Twenty-nine healthy women (mean age, 39.7 years) without symptoms of lower genital tract infection who visited the Hospital Health Center were selected as a control group. The Institutional Review Board approved this study.
For RNA/DNA isolation, a QIAamp RNA/DNA mini kit (Qiagen, Hilden, Germany) was used according to the manufacturer's instructions. Vaginal swabs and EPS specimens were swirled in lysis buffer containing 1% Triton X-100, 0.5% Tween 20, and 1 mmol EDTA. After mixing the samples with 200 µl buffer AL (Qiagen) and 20 µl proteinase K, the samples were incubated for 30 min at 56℃ followed by 15 min at 95℃. For synthesis of cDNA, reverse transcription was carried out by using the Reverse AIDTM First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). Pre-PCR products were stored at -20℃ until use.
Ten microliters of each bacterial RNA was denatured at 80℃ for 3 min; mixed with a master mix consisting of 4 µl of 5x RT-buffer, 2 µl of dNTPs, 1 µl of RNase inhibitor, 1 µl of reverse random primer, 1 µl dithiothreitol (DTT), and 1 µl of reverse transcriptase; and incubated for 90 min at 37℃. After inactivation of reverse transcriptase by incubation at 94℃ for 2 min, cDNAs were processed immediately for amplification.
cDNAs (3 µl) were mixed with a PCR premix consisting of 10x PCR buffer, 1 µl of forward primer (5'-acaatggtggtgacagtggg-3'), and 1 µl of reverse primer (5'-cgggtaaaaccaactcccat-3') (Table 1). Forty cycles were carried out at 94℃ for 30 s and 60℃ and 72℃, each for 90 s. Then the PCR mix (10 µl) was subjected to 4% agarose gel electrophoresis at 100 V for 30 min and nucleic acid bands were visualized by ethidium bromide staining.
A multiplex PCR has been designed for simultaneous detection of N. gonorrhea, C. trachomatis, U. urealyticum, M. hominis, T. vaginalis, and M. genitalium. The Seeplex Sexually Transmitted Disease Detection assay (Seegene Inc., Seoul, Korea) uses two separate primer mixes and can detect the DNA for 6 types of sexually transmitted pathogens (Table 1).
In order to detect nanobacterial RNA in EPS and vaginal swabs, RT-PCR was performed with primers specifically designed for direct detection of nanobacterial genomic elements. Fig. 1 shows the results of agarose gel electrophoresis of RT-PCR products (band at 208 bp). In EPS samples, the detection rate of NB in patients with CP/CPPS was 9.1%, and all of the 5 healthy volunteers were negative. There was no significant difference in the detection rate of NB by RT-PCR between the two groups (p=0.48). Nine (5.7%) of 157 vaginitis patients who showed positive results on RT-PCR for NB in vaginal swabs and all of the 29 healthy volunteers were negative. There was no significant difference in the detection rate of NB by RT-PCR between the two groups (p=0.19).
A total of 11 patients with CP/CPPS were included in the study. Six patients (54.5%) tested positive on EPS. The associations observed among the 7 microorganisms in the group of symptomatic men analyzed are summarized in Table 2. Monoinfection was detected in 5 of 6 positive patients (65.3%). The majority of monoinfections were C. trachomatis. One patient was co-infected with two organisms (16.7%). NB were co-infected with C. trachomatis.
A total of 157 vaginitis patients were included in the study. Seventy-five patients (47.8%) tested positive on vaginal swabs. The associations observed among the 7 microorganisms in the group of symptomatic women analyzed are summarized in Table 3. Monoinfection was detected in 49 of 75 positive patients (65.3%). The majority of monoinfections were C. trachomatis and U. urealyticum. Nineteen patients were co-infected with two organisms (25.3%), 5 patients were co-infected with three organisms (6.7%), and 2 patients were co-infected with 4 organisms (2.7%). Our data demonstrate the association in vivo between monoinfection of nanobacteria and vaginitis (6.7%). NB were co-infected with C. trachomatis, U. urealyticum, and M. hominis.
NB have recently been described as novel microorganisms characterized by a small size (0.2 to 0.5 µ) with a 16S rRNA gene sequence and slow growth and a doubling time of about 3 days. They are gram-negative, have a unique structure and apparent nucleic acid, and their growth in vitro is best inhibited by tetracycline [10]. NB exist in blood, urine, and other organs and tissues [2]. Raoult et al and Young et al thought that NB were mineral-fetuin complexes or pleomorphic mineralo-protein complexes [11,12]; nevertheless, they could not exclude the possibility that NB are pathogenic microorganisms. In the clinical situation, NB may initiate kidney stone formation [5]. They have been found in periodontal disease [13], calcified human valves [4], and in urine and bladder tissue samples of patients with interstitial cystitis/painful bladder syndrome [14] and have been shown to participate in the clinical pathological process of those diseases.
In addition to the culture method, several other diagnostic tools have been developed for identification of NB. One of the most powerful methods is transmission electron microscopy (TEM); however, this method requires very expensive equipment. Conventional NB culture and antigenic detection do not require expensive equipment; however, these methods are often time consuming with cumbersome procedures. For DNA sequencing, genomic RNA is isolated from NB cultures, which is similar to the above methods. Therefore, we developed a primer for conventional RT-PCR, which makes it possible to find NB from uncultured direct specimens. This method was superior to TEM, conventional NB culture, and DNA sequencing of isolated NB cultures. Our method was rapid, simple, low in cost, and easily available because of the use of uncultured specimens and conventional RT-PCR.
Zhou et al found a 62.5% and 12.5% NB-positive rate in cultured EPS and urine samples, respectively, after prostatic massage in men with type III prostatitis [7]. Another study found indirect evidence of NB on antigen and antibody by using enzyme-linked immunosorbent assay (ELISA) in 40% of urine samples and 0% of EPS in patients with CP/CPPS [15]. Shen et al postulated that the pathogenesis of prostatic calculi involves a certain mechanism: 1) NB form calcifications and mineral deposition cores; 2) the prostatic epithelial membrane is damaged by nanobacterial infection, causing exposure of tissue components that may form crystal cores; 3) NB mix with prostatic secretions; 4) with urine backflow, high metabolite concentrations result. Shen et al concluded that NB might be an important etiological factor for type III prostatitis [16]. In our 11 direct EPS samples, the detection rate of NB in patients with CP/CPPS was 9.1% and NB were co-infected with C. trachomatis. C. trachomatis was most commonly detected with CP/CPPS and the frequency of co-infection with NB was higher than that for other infectious organisms. In our study, the NB-positive rate in direct EPS was lower than 62.5% with cultured EPS and higher than 0% with the ELISA method. In our opinion, because we used only the direct EPS samples, and not cultured EPS samples, the NB-positive rate was low. The culture method showed a high positive rate but required a minimum of 5 weeks and had opportunity for contamination. The ELISA method for detection of antibody showed a positive rate that was too low. RT-PCR for NB has the advantages of being rapid, simple, low in cost, and easily available. The sequences obtained were confirmed as NB by comparison with those in the GenBank (National Center for Biotechnology Information) database by using the Basic Local Alignment Search Tool (BLAST). However, when applying molecular assays as a routine diagnostic test, one should be aware of false-positives resulting from the amplified method. Also, clinical diagnosis by PCR only may be inaccurate, because vaginitis and prostatitis caused by NB cannot be distinguished from that caused by the normal flora and contamination, and the positive results of PCR are not always the cause of the disease.
According to current opinion, type III prostatitis is probably related to nanobacterial infection, mainly because NB have been shown to cause multiple organic infections, especially urologic infections. Also, after anti-NB treatment, the NB-positive rate decreased significantly, and the patients' symptoms resolved [7]. It is important that we precisely identify the cause of infection and provide the correct treatment. Therefore, we attempted to develop a rapid, simple, low-cost, and easily available method for use with uncultured specimens.
Vaginal infection encompasses a broad range of symptoms, ranging from a state of severe inflammation and irritation with a frothy malodorous discharge, pain, and dyspareunia to an asymptomatic carrier state, which is estimated to constitute up to 50% of cases [17]. Infections by U. urealyticum, M. hominis, and T. vaginalis during pregnancy can lead to premature rupture of the placental membranes, premature labor, and low-birth-weight infants [18]. In this study, 9 (5.7%) of 157 vaginitis patients showed positive results in RT-PCR for NB in vaginal swabs and all of the 29 healthy volunteers were negative. There was no significant difference in the detection rate for NB by RT-PCR between the two groups (p=0.19). However, we found five patients who were not positive for N. gonorrhea, C. trachomatis, U. urealyticum, M. hominis, T. vaginalis, or M. genitalium who were only infected with NB. Our data suggest that nanobacteria may be an etiological factor for vaginitis. The prevalence of U. urealyticum among the four patients with NB co-infection was 75%; the presence of U. urealyticum might raise suspicion for nanobacterial infection. A symbiotic association between NB and U. urealyticum was implied; however, the number of subjects co-infected with NB was too small, which was a limitation of the study. Unfortunately, physicians could not determine whether nanobacteria were the cause of infection because it would take too much time to detect the nanobacteria and it would not result in appropriate treatment.
Although the controversies about whether NB are living organisms are continuing, the results of our study suggest that conventional RT-PCR for NB is rapid, simple, low in cost, and easily available for the detection of NB and that NB may be an etiological factor for vaginitis and prostatitis. However, there were several limitations to our study. First, there were a significant number of patients, both with CP/CPPS and vaginitis, for whom the positive results of NB testing were not available. Second, we could not compare our results with the results of the culture method and TEM. Finally, we did not attempt to correlate the clinical presentation of our patients with their test results. It was not the purpose of our study.
We found that conventional RT-PCR for NB was rapid, simple, low in cost, and easily available for the detection of NB and that NB may be a possible etiological factor for vaginitis and CP/CPPS. The prevalence of U. urealyticum among the four patients with NB co-infection was 75%; the presence of U. urealyticum may therefore raise suspicion for nanobacterial infection. Physicians may want to consider NB as the cause of infection and attempt to provide treatment with an appropriate drug.
Figures and Tables
FIG. 1
Reverse transcriptase polymerase chain reaction for nanobacteria. M: size marker, Pt: patient with vaginitis, NC: negative control, bp: base pair.
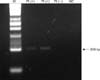
TABLE 1
Target for detection of seeplex sexually transmitted disease detection assay by multiplex PCR assays and conventional primers for nanobacteria by RT-PCR

Notes
References
1. Millán-Rodríguez F, Palou J, Bujons-Tur A, Musquera-Felip M, Sevilla-Cecilia C, Serrallach-Orejas M, et al. Acute bacterial prostatitis: two different sub-categories according to a previous manipulation of the lower urinary tract. World J Urol. 2006. 24:45–50.
2. Wood HM, Shoskes DA. The role of nanobacteria in urologic disease. World J Urol. 2006. 24:51–54.
3. Fortenberry JD, Brizendine EJ, Katz BP, Wools KK, Blythe MJ, Orr DP. Subsequent sexually transmitted infections among adolescent women with genital infection due to Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis. Sex Transm Dis. 1999. 26:26–32.
4. Bratos-Pérez MA, Sánchez PL, García de Cruz S, Villacorta E, Palacios IF, Fernández-Fernández JM, et al. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008. 29:371–376.
5. Ciftçioglu N, Björklund M, Kuorikoski K, Bergström K, Kajander EO. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 1999. 56:1893–1898.
6. Kajander EO, Ciftçioglu N. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci U S A. 1998. 95:8274–8279.
7. Zhou Z, Hong L, Shen X, Rao X, Jin X, Lu G, et al. Detection of nanobacteria infection in type III prostatitis. Urology. 2008. 71:1091–1095.
8. Hudelist G, Singer CF, Kubista E, Manavi M, Mueller R, Pischinger K, et al. Presence of nanobacteria in psammoma bodies of ovarian cancer: evidence for pathogenetic role in intratumoral biomineralization. Histopathology. 2004. 45:633–637.
9. Litwin MS, McNaughton-Collins M, Fowler FJ Jr, Nickel JC, Calhoun EA, Pontari MA, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999. 162:369–375.
10. Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004. 287:H1115–H1124.
11. Raoult D, Drancourt M, Azza S, Nappez C, Guieu R, Rolain JM, et al. Nanobacteria are mineralo fetuin complexes. PLoS Pathog. 2008. 4:e41.
12. Young JD, Martel J, Young D, Young A, Hung CM, Young L, et al. Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo-protein complexes and as precursors of putative nanobacteria. PLoS One. 2009. 4:e5421.
13. Ciftçioğlu N, McKay DS, Kajander EO. Association between nanobacteria and periodontal disease. Circulation. 2003. 108:e58–e59.
14. Zhang QH, Shen XC, Zhou ZS, Chen ZW, Lu GS, Song B. Decreased nanobacteria levels and symptoms of nanobacteria- associated interstitial cystitis/painful bladder syndrome after tetracycline treatment. Int Urogynecol J Pelvic Floor Dysfunct. 2010. 21:103–109.
15. Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol. 2005. 173:474–477.
16. Shen X, Ming A, Li X, Zhou Z, Song B. Nanobacteria: a possible etiology for type III prostatitis. J Urol. 2010. 184:364–369.
17. Soper D. Trichomoniasis: under control or undercontrolled? Am J Obstet Gynecol. 2004. 190:281–290.
18. Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis. 2006. 25:562–569.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download