Abstract
Purpose
Many studies about efficacy of alpha blocker to Chronic prostatitis/ chronic pelvic pain syndrome (CP/CPPS) have shown variable results. The aim of this study was to confirm the efficacy of alpha blocker in young and middle aged patients with CP/CPPS to exclude the effect of benign prostatic hyperplasia.
Materials and Methods
Fifty seven men with CP/CPPS were randomized in a single-blind fashion, to receive either; tosufloxacin (450mg/d) (group 1; 15 patients), or; tosufloxacin (450mg/d) and alfuzosin (10mg/d) (group 2; 42 patients) for 2 months. The NIH chronic prostatitis symptom index (NIH-CPSI), International Prostate Symptom Score (IPSS) and International Index of Erectile Function-5 (IIEF-5) were used to grade the symptoms and the quality of life (QoL) impact at the start and 1 and 2 months into the study.
Results
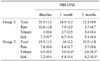
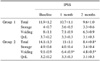
There was no significant difference between group 1 and group 2 in relation to age, duration and sub-factor scores of IPSS, NIH-CPSI and IIEF-5 at the baseline. No statistically significant difference in the NIH-CPSI total score was seen, but the urinary and QoL factors in group 2 showed greater improvement. A statistically significant difference was seen in the IPSS total score, especially, obstructive factor in group 2 showed greater improvement. The IIEF-5 total score was seen more increase, but it wasn't significant.
Figures and Tables
Table 1
Comparison of the NIH-CPSI results in the pre-treatment and post-treatment period (1-2 months)
References
1. Collins MM, Stafford RS, O'Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998. 159:1224–1228.
2. Collins MM, Meigs JB, Barry MJ, Walker Corkery E, Giovannucci E, Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002. 167:1363–1366.
3. Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, Alexander RB, Litwin MS, et al. Demographic and clinical characteristics of men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol. 2002. 168:593–598.
4. McNaughton Collins M, Pontari MA, O'Leary MP, Calhoun EA, Santanna J, Landis JR, et al. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001. 16:656–662.
5. Krieger JN, Nyberg L, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999. 282:236–237.
6. Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology. 1999. 54:229–233.
7. Alexander RB, Propert KJ, Schaeffer AJ, Landis JR, Nickel JC, O'Leary MP, et al. Chronic Prostatitis Collaborative Research Network. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004. 141:581–589.
8. Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, et al. Initial, long-term, and durable responses to terazosin, placebo, or other therapies for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2004. 64:881–886.
9. Collins MM, O'Leary MP, Barry MJ. Prevalence of bothersome genitourinary symptoms and diagnoses in younger men on routine primary care visits. Urology. 1998. 52:422–427.
10. Ku JH, Kim ME, Lee NK, Park YH. The prevalence of chronic prostatitis-like symptoms in young men: a community-based survey. Urol Res. 2001. 29:108–112.
11. Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE. Intraprostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol. 1982. 54:729–731.
12. Nickel JC, Narayan P, McKay J, Doyle C. Treatment of chronic prostatitis/chronic pelvic pain syndrome with tamsulosin: a randomized double blind trial. J Urol. 2004. 171:1594–1597.
13. Mehik A, Alas P, Nickel JC, Sarpola A, Helstrom PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003. 62:425–429.
14. de la Rosette JJ, Karthaus HF, van Kerrebroeck PE, de Boo T, Debruyne FM. Research in 'prostatitis syndromes': the use of alfuzosin (a new alpha 1-receptor-blocking agent) in patients mainly presenting with micturition complaints of an irritative nature and confirmed urodynamic abnormalities. Eur Urol. 1992. 22:222–227.
15. Lacquaniti S, Destito A, Servello C, Candidi MO, Weir JM, Brisinda G, et al. Terazosine and tamsulosin in non bacterial prostatitis: a randomized placebo-controlled study. Arch Ital Urol Androl. 1999. 71:283–285.
16. Lee JH, Jeon JS, Cho IR. Characteristic symptoms of chronic prostatitis/chronic pelvic pain syndrome. Korean J Urol. 2002. 43:852–857.
17. Litwin MS. A review of the development and validation of the National Institutes of Health Chronic Prostatitis Symptom Index. Urology. 2002. 60:14–18.
18. Zhang JG, Wang YL, Ren XQ, Gao ZW, Cheng YH. Sexual function of men with symptomatic benign prostatic hyperplasia and effect of tamsulosin. Zhonghua Nan Ke Xue. 2006. 12:723–725.
19. Lee SW, Liong ML, Yuen KH, Liong YV, Krieger JN. Chronic prostatitis/chronic pelvic pain syndrome: role of alpha blocker therapy. Urol Int. 2007. 78:97–105.
20. Jung YH, Kim JG, Cho IR. The efficacy of terazosin in the management of chronic pelvic pain syndrome (CPPS): comparison between category IIIa and IIIb. Korean J Urol. 2006. 47:1191–1196.
21. Schaeffer AJ. National Institute of Diabetes and Digestive and Kidney Diseases of the US National Institutes of Health. NIDDK-sponsored chronic prostatitis collaborative research network (CPCRN) 5-year data and treatment guidelines for bacterial prostatitis. Int J Antimicrob Agents. 2004. 24:Suppl 1. S49–S52.
22. Fall M, Baranowski AP, Fowler CJ, Lepinard V, Malone-Lee JG, Messelink EJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2004. 46:681–689.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download