Abstract
Purpose:
The aim of this study was to investigate the relationship of cyclooxygenase (CoX)-2 expression and microvessel density (MVD), a reflection of angiogenesis, with prognosis in patients with transitional cell carcinoma of the upper urinary tract (TCC-UUT).
Materials and Methods:
Formalin-fixed, paraffin-embedded tissue sections of TCC-UUT from 91 patients, who had undergone radical nephroureterectomy, were examined immunohistochemically using antibodies against CoX-2 and CD34. MVD was determined with CD34-stained slides. The expression patterns of COX-2 and MVD were compared with the clinicopathological variables.
Results:
The COX-2 expression was significantly correlated with T stage (p=0.002), N stage (p=0.010), and grade (p=0.027). MVD was also significantly correlated with T stage (p く 0.001), N stage (p=0.002), and grade (p=0.001). The COX-2 expression was significantly correlated with MVD (p=0.001). The survival rate of patients with COX-2 positive tumors or high MVD was significantly lower than that of patients with COX-2 negative tumors or low MVD, respectively (p=0.0013, p=0.0312). Univariate analyses identified T stage, N stage, grade, COX-2 expression, and MVD as significant prognostic factors for cancer-specific survival; multivariate analyses indicated that T stage was the only independent prognostic factor.
Conclusions:
The increased expression of COX-2 and MVD is associated with a worse prognosis in TCC-UUT. The COX-2 expression is correlated with MVD. These results suggest that COX-2 may play an important role in the progression of TCC-UUT and angiogenesis may be affected by COX-2 during the progression of TCC-UUT.
REFERENCES
1.Iborra I., Solsona E., Casanova J., Ricos JV., Rubio J., Climent MA. Conservative elective treatment of upper urinary tract tumors: a multivariate analysis of prognostic factors for recurrence and progression. J Urol. 2003. 169:82–5.

2.Cho KS., Cho NH., Choi YD. Pattern of recurrence and the prognostic factors of upper urinary tract transitional cell carcinoma. Korean J Urol. 2006. 47:124–30.

3.Miyata Y., Kanda S., Nomata K., Eguchi J., Kanetake H. Expression of cyclooxygenase-2 and EP4 receptor in transitional cell carcinoma of the upper urinary tract. J Urol. 2005. 173:56–60.

4.Langner C., Hutterer G., Chromecki T., Winkelmayer I., Rehak P., Zigeuner R. pT classification, grade, and vascular invasion as prognostic indicators in urothelial carcinoma of the upper urinary tract. Mod Pathol. 2006. 19:272–9.

5.Vane JR., Bakhle YS., Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998. 38:97–120.

6.Park MK., Chung JI., Jung SJ., Choi SH. Expression of cyclooxygenase-2 in urothelial carcinoma of the human urinary bladder. Korean J Urol. 2005. 46:1155–60.
7.Pruthi RS., Derksen E., Gaston K. Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol. 2003. 169:2352–9.

8.Oku S., Higashi M., Imazono Y., Sueyoshi K., Enokida H., Kubo H, et al. Overexpression of cyclooxygenase-2 in high-grade human transitional cell carcinoma of the upper urinary tract. BJU Int. 2003. 91:109–14.

9.Sharma S., Sharma MC., Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005. 46:481–9.

10.Blood CH., Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990. 1032:89–118.

11.Weidner N., Carroll PR., Flax J., Blumenfeld W., Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993. 143:401–9.
12.Inoue K., Kamada M., Slaton JW., Fukata S., Yoshikawa C., Tamboli P, et al. The prognostic value of angiogenesis and metastasis-related genes for progression of transitional cell carcinoma of the renal pelvis and ureter. Clin Cancer Res. 2002. 8:1863–70.
13.Zhang X., Kong C., Takenaka I. Evaluation of cell proliferation, apoptosis, and angiogenesis in transitional cell carcinoma of the renal pelvis and ureter. Urology. 2001. 57:981–5.

14.Greene FL., Page DL., Fleming ID., Fritz AG., Balch CM., Haller DG, et al. AJCC cancer staging manual. 6th ed.New York: Springer-Verlag;2002. ;329-31.
15.Epstein JI., Amin MB., Reuter VR., Mostofi FK. The World Health Organization/Intemational Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998. 22:1435–48.
16.Sinicrope FA., Ruan SB., Cleary KR., Stephens LC., Lee JJ., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–41.
17.Mohammed SI., Knapp DW., Bostwick DG., Foster RS., Khan KN., Masferrer JL, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999. 59:5647–50.
18.Gurocak S., Sozen S., Erdem 0., 〇zkan S., Kordan Y., Alkibay T, et al. Relation between cyclooxygenase-2 expression and clinicopathologic parameters with patient prognosis in transitional cell carcinoma of the bladder. Urol Int. 2006. 76:51–6.

19.Shariat SF., Matsumoto K., Kim J., Ayala GE., Zhou JH., Jian W, et al. Correlation of cyclooxygenase-2 expression with molecular markers, pathological features and clinical outcome of transitional cell carcinoma of the bladder. J Urol. 2003. 170:985–9.

20.Shirahama T., Arima J., Akiba S., Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder Cancer. 2001. 92:188–93.
21.Friedrich MG., Toma MI., Petri S., Huland H. Cyclooxygenase-2 promotes angiogenesis in pTa/Tl urothelial bladder carcinoma but does not predict recurrence. BJU Int. 2003. 92:389–92.
Fig. 1.
Immunohistochemical staining for cyclooxygenase-2 (COX-2) in transitional cell carcinoma of the upper urinary tract tissues (x400). (A) Cancer cells are not stained (COX-2 intensity 0). (B) Smooth muscle cells are stained as an internal control. Cancer cells are stained weakly compared to an internal control (intensity 1). (C) Cancer cells are stained in same intensity as internal control (intensity 2). (D) Cancer cells are strongly stained for COX-2 (intensity 3).

Fig. 2.
Kaplan-Meier cancer-specific survival curves according to cyclooxygenase-2 (COX-2) expression (A) or microvessel density (MVD) (B). The survival rate of patients with COX-2 positive tumors or high MVD was significantly lower than that of patients with COX-2 negative tumors or low MVD, respectively (p=0.0013, p=0.0312).
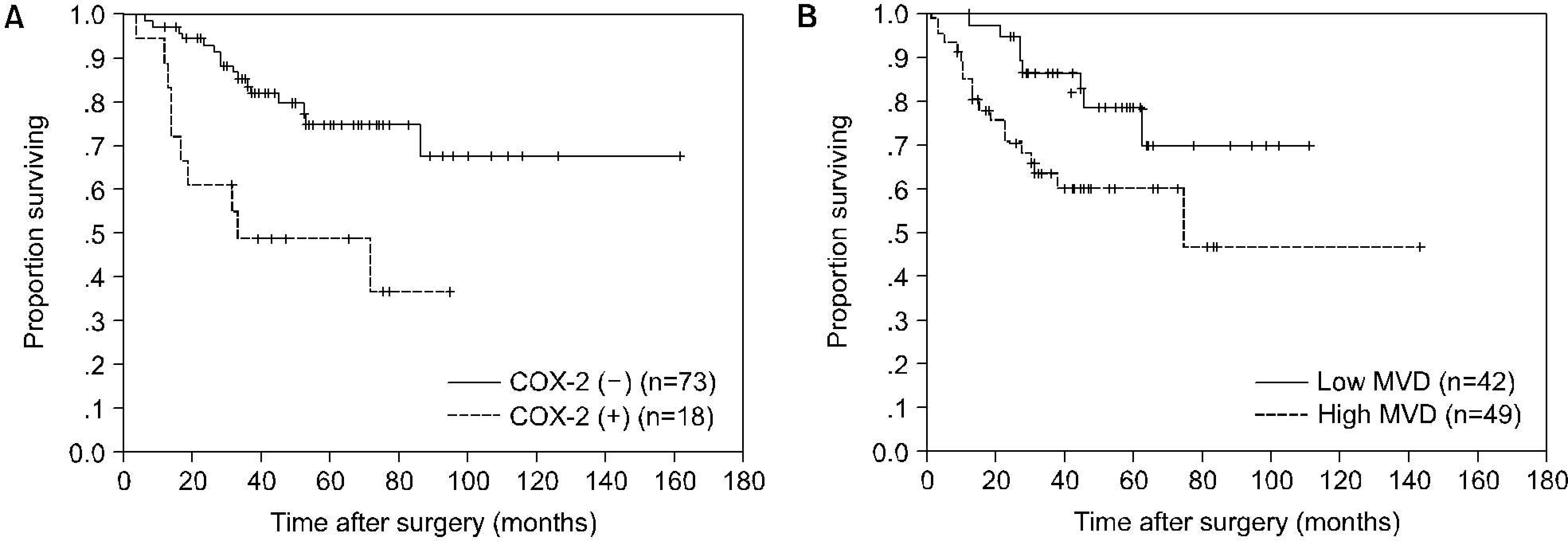
Table 1.
Clinicopathological data
Table 2.
Relationship between COX-2 expression or microvessel density and clinicopathological variables
Table 3.
Univariate and multivariate survival analyses




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download