Abstract
Insulin resistance is a hallmark of metabolic syndrome, including type 2 diabetes mellitus and obesity which are characterized by endothelial dysfunction. The endothelial dysfunction leads to erectile dysfunction (ED). Thus, understanding of insulin resistance is mandatory for urologists to understand a pathophysiology of ED. There are two distinct features of insulin signal transduction pathway. One is phosphatidylinositol 3-ki- nase-dependent pathway which regulates glucose uptake in skeletal muscle and nitric oxide (NO) production and vasodilation in vascular endo- thelium. In cavernous smooth muscles, insulin also induces the endothe- lium-dependent relaxation, however it may emanate from the direct inhibition of ᄂ-type Ca2+ channels by prostacyclin in addition to NO production. The other one is mitogen-activated protein (MAP)-kinase pathway which regulates growth and mitogenesis and controls secretion of endo- thelin-1 in vascular endothelium. Stimulation of sympathetic activity by insulin may also contribute to hemodynamic regulation. A shift in balance between vasoconstrictor and vasodilator actions of insulin may be an important factor in the vascular pathophysology of insulin resistance, leading to ED. Hyperglycemia, elevated free fatty acid levels, and proin- flammatory states share mechanisms between insulin resistance and endothelial dysfunction. There is evidence to suggest that testosterone is an important regulator of insulin sensitivity in men and hypotestoste- ronemia may have a role in the pathogenesis of insulin-resistant states. (Korean J Urol 2006;47:917-927)
REFERENCES
1.Alberti KG., Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1—diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998. 15:539–53.
2.Western Pacific Regional Office of the World Health Organization. The International Obesity Task Force: The Asia-Pacific perspective: redefining obesity and its treatment. Sidney: Health Communications Australia;2000. http://www.obe-sityasiapacific.com.
3.Hellmich M., Evers T., Kubin M., Merchant S., Lehmacher W., Engelmann U, et al. Development and validation of a risk score for somatic erectile dysfunction: combined results from three cross-sectional surveys. Eur Urol. 2005. 48:495–502.

4.Kim JH., Shim BS., Hong YS. The relating factors of metabolic syndrome to benign prostatic hyperplasia. Korean J Urol. 2005. 46:1046–50.
5.Salitel AR., Kahn CR. Insulin signaling and the regulation of glucose aM lipid metabolism. Nature. 2001. 414:799–806.
6.Nystrom FH., Quon MJ. Insulin signalling: metabolic pathways and mechanism for specificity. Cell Signal. 1999. 11:5630–74.
7.Zeng G., Qoun MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin: direct measurement in vascular endothelial cells. J Clin Invest. 1996. 98:894–8.

8.Zeng G., Nystrom FH., Ravichandran IV., Cong IN., Kirby M., Mostowski H, et al. Roles for insulin recptor. PI 3-kinase and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000. 101:1539–45.
9.Montagnani M., Ravichandran IV., Chen H., Esposito DL., Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002. 16:1931–42.

10.Montagnani M., Chen H., Barr VA., Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Aky at Ser (1179). J Biol Chem. 2001. 276:30392–8.
11.Grubb B., Snarr JF. Effect of flow rate and glucose concentration on glucose uptake rate by the rat limb. Proc Soc Exp Biol Med. 1977. 154:33–6.

12.Potenza MA., Marasciulo FL., Chieppa DM., Brigiani GS., Formoso G., Quon MJ, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005. 289:H813–22.

13.Anderson EA., Hoffman RP., Balmon TW., Sinkey CA., Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991. 87:2246–52.

14.Sartori C., Traeb L., Nicod P., Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition of vascular actions of insulin in humans. Hypertension. 1999. 34:586–9.
15.Creager MA., Liang CS., Co任man JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther. 1985. 235:709–14.
16.Vierhapper H. Effect of exogenous insulin on blood pressure regulation in healthy and diabetic subjects. Hypertension. 1985. 7:49–53.

17.Montagnani M., Golovchenko I., Kim I., Koh GY., Goalstone ML., Mundhekar AN, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002. 277:1794–9.

18.Myung SC., Keum EM., Park SY., Lee MY., Kim SC. Vasomotor action of insulin on the rabbit normal cavernous smoodi muscle. Eur J Pharmacol. 2006. 536:142–7.
19.Klein R., Klein BE., Lee KE., Moss SE., Cruickshanks KJ. Prevalence of self-reported erectile dysfunction in people with long-term IDDM. Diabetes Care. 1996. 19:135–41.

20.Kuboki K., Jiang ZY., Takahara N., Ha SW., Igarashi M., Yamauchi T, et al. Regulation of endothelial constitutive nitiric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000. 101:676–81.
21.Miller AW., Tulbert C., Puskar M., Busija DW. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002. 40:78–82.

22.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. 114:1752–61.

23.Gao Z., Hwang D., Bataille I., Lefevre M., York D., Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002. 277:48115–21.
24.Hirosumi J., Tuncman G., Chang L., Gorgun CZ., Uysal KT., Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002. 420:333–6.

25.Salt JP., Morrow VA., Brandie FM., Connell JM., Petrie JR. High glucose inhibits insulin-stimulated nitric oxide production without reducing endothelial nitric-oxide synthase Seri 177 phosphorylation in human aortic endothelial cells. J Biol Chem. 2003. 278:18791–7.
26.Quagliaro L., Piconi L., Assaloni R., Da Ros R., Maier A., Zuodar G, et al. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005. 183:259–67.

27.Quagliaro L., Piconi L., Assaloni R., Martinelli L., Motz E., Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD (P)H-oxidase activation. Diabetes. 2003. 52:2795–804.
28.Espesito K., Nappo F., Margella R., Giugliano D., Giugliano F., Ciotoal M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002. 106:2067–72.
29.Nishikawa T., Edelstein D., Du XL., Yamagishi S., Matsumura T., Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000. 404:787–90.
30.Wautier MP., Chappey O., Corda S., Stem DM., Schmidt AM., Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001. 280:E685–94.

31.Hofmann MA., Drury S., Fu C., Qu W., Taguchi A., Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999. 97:889–901.
32.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006. 290:El–8.

33.Veerababu G., Tang J., Hoffman RT., Daniels MC., Hebert LF Jr., Crook ED, et al. Overexpression of glutamine: fructose-e-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance. Diabetes. 2000. 49:2070–8.
34.Schulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000. 106:171–6.
35.Steinberg HO., Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002. 45:623–34.

36.Inoguchi T., Li P., Umeda F., Yu HY., Kakimoto M., Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NADPH oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–45.

37.O' Donnell VB., Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001. 88:12–21.
38.Ajuwon KM., Spurlock MI. Palmitate activates die NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-1, 1 adipocytes. J Nutr. 2005. 135:1841–6.
39.Jove M., Planavila A., Laguna JC., Vazquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology. 2005. 146:3087–95.
40.Boden G., She P., Mozzoli M., Cheung P., Gumireddy K., Reddy P, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kB pathway in rat liver. Diabetes. 2005. 54:3458–65.
41.Gao Z., Zhang X., Zuberi A., Hwang D., Quon MJ., Lefevre M, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004. 18:2024–34.

42.Kim F., Tysseling KA., Rice J., Pham M., Haji L., Gallis BM, et al. Free fatty acid impairment of nitiric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vase Biol. 2005. 25:989–94.
43.Chavez JA., Knotts TA., Wang LP., Li G., Dobrowsky RT., Florant GL, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003. 278:10297–303.

44.Igarashi J., Thatte HS., Prabbakar P., Golan DE., Michel T. Calcium-independent activation of endodielial nitric oxide synthase by ceramide. Proc Natl Acad Sci USA. 1999. 96:12583–8.
45.Li H., Junk P., Huwiler A., Burkhardt C., Wallerath T., Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002. 106:2250–6.
46.Nguyen MT., Satoh H., Favelyulds S., Babendure JI., Imamura T., Shodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005. 280:35361–71.
47.Gao Z., Zuberi A., Quon MJ., Dong Z., Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate I in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003. 278:24944–50.
48.de Alvaro C., Teruel T., Hernandez R., Lorenzo M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner J Biol Chem. 2004. 279:17070–8.
49.Li X., Commane M., Jiang Z., Stark GR. IL-1-mediated NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK). Proc Natl Acad Sci USA. 2001. 98:4461–5.
50.Kim F., Gallis B., Corson MA. TNF-alpha inhibits flow ad insulin signaling leading to NO production in aortic endothelial cells. Am J Physiol Cell Physiol. 2001. 280:C1057–65.
51.Anderson HD., Rahmutula D., Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promotor activity in bovine aortic endothelial cells. J Biol Chem. 2004. 279:963–9.
52.Min JK., Kim YM., Kim SW., Kwon MC., Kong YY., Hwang IK, et al. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-de-pendent NF-kappaB activation in endothelial cells. J Immunol. 2005. 175:531–40.
53.Peng HB., Libby P., Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995. 270:14214–9.
54.De Caterina P., Libby P., Peng HB., Thannickal VJ., Rajavahisth TB., Gimbrone MA Jr, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995. 96:60–8.

55.Saito M., khimitsu T., Minami J., Ono H., Ohrui M., Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003. 167:73–9.

56.Jialal I., Devaraj S., Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004. 44:6–11.

57.Venugopal SK., Devaraj S., Yuhanna I., Shaul P., Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002. 106:1439–41.

58.Wang CH., Li SH., Weisel RD., Fedak PW., Dumont AS., Szmitko P, et al. C-reactive protein upregulates angiotensin type I receptors in vascular smooth muscle. Circulation. 2003. 107:1783–90.
59.Pasceri V., Cheng JS., Willerson JT., Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-athrosclerosis drugs. Circulation. 2001. 103:2531–4.
60.Pasceri V., Willerson JT., Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000. 102:2165–8.

61.Jiang ZY., Lin YW., Clemont A., Feener EP., Hein KD., Igarashi M, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker rats. J Clin Invest. 1999. 104:447–57.
62.Kuboki K., Jiang ZY., Takahara N., Ha SW., Igarashi M., Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000. 101:676–81.
63.Federici M., Pandolfi A., De Filippis EA., Pellegrini G., Menghini R., Lauro D, et al. G972R IRS-1 variant impairs insulin regulation of endothelial nitric oxide synthase in cultured human endothelial cells. Circulation. 2004. 109:399–405.

64.Burnett AL. Novel nitric oxide signaling mechanisms regulate the erectile response. Int J Impot Res. 2004. 16(Suppl l):S15–9.

65.Piatti PM., Monti LD., Conti M., Baruffaldi L., Galli L., Phan CV, et al. Hypertriglyceridemia and hyperinsulinemia are potent inducers of endothelin-1 release in humans. Diabetes. 1996. 45:316–21.

66.Folli F., Kahn CR., Hansen H., Bouchie JL., Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role of serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 1997;. 100:2158.
67.Andreozzi F., Laratta E., Sciacqua A., Perticone F., Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004. 94:1211–8.
68.Rajagopalam S., Kurz S., Munzel T., Tarpey M., Freeman BA., Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996. 97:1916–23.
69.Pastore I., Tessitore A., Martinotti S., Toniato E., Alesse E., Bravi MC, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999. 100:1646–52.

70.Desideri G., Ferri C., Bellini C., De Mattia G., Santucci A. Effects of ACE inhibition on spontaneous and insulin-stimulated endothelin-1 secretion: in vitro and in vivo studies. Diabetes. 1997. 46:81–6.

71.Park JK., Kim SZ., Kim SH., Park YK., Cho KW. Renin angiotensin system in rabbit corpus cavemosum: functional characterization of angiotensin II receptors. J Urol. 1997. 158:653–8.
72.Yu JG., Javorschi S., Hevener AL., Kruszynska YT., Norman RA., Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002. 51:2968–74.

73.Goldstein BJ., Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004. 89:2563–8.

74.Chen H., Montagnami M., Funahashi T., Shimomura I., Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003. 278:45021–6.

75.Yamauchi T., Hara K., Kubota N., Terauchi Y., Tobe K., Froguel P, et al. Dual roles of adiponectin/Acrp30 in vivo as an antidiabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord. 2003. 3:243–54.
76.Borissova AM., Tankova T., Kamenova P., Dakovska L., Kovacheva R., Kitilov G, et al. Effect of hormone replacement therapy on insulin secretion and insulin sensitivity in postmenopausal diabetic women. Gynecol Endocrinol. 2002. 16:67–74.

77.Holmang A., Larsson BM., Brzezinsak Z., Bjomtorp P. Effects of short term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol. 1992. 262:E851–5.
78.Malkin Cl., Pugh PJ., Iones TH., Channer KS. Testosterone for secondary prevention in men with ischemic heart disease. Ouart J Med. 2003. 96:521–9.
79.Simon D., Preziosi P., Barrett-Connor F., Roger M., Saint-Paul M., Nahoul K, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: The Telecom Study. Diabetologia. 1992. 35:173–7.

80.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992. 117:807–11.

81.Smith MR., Lee H., Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006. 91:1305–8.

82.Stellato RK., Feldman HA., Hamdy O., Horton FS., McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts Male Aging Study. Diabetes Care. 2000. 23:490–4.

83.Abate N., Haffiier SM., Garg A., Peshock RM., Grundy SM. Sex steroid hormones, upper body obesity, and insulin resistance. J Clin Endocrinol Metab. 2002. 87:4522–7.

84.Simon D., Charles MA., Nahoul K., Orssaud G., Kremski J., Hully V, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J Clin Endocrinol Metab. 1997. 82:682–5.

85.Andersson B., Marin P., Lissner I., Vermeulen A., Bjomtorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994. 17:405–11.

86.Pitteloud N., Hardin M., Dwyer AA., Valassi E., Yialamas M., Elahi D, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005. 90:2636–41.

87.Pitteloud N., Mootha VK., Dwyer AA., Hardin M., Lee H., Eriksson KF, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005. 28:1636–42.

88.Roden G., Jadali F., White J., Liang Y., Mozzoli M., Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991. 88:960–6.
89.Mootha VK., Lindgren CM., Eriksson KF., Subramanian A., Silhag S., Lehar J, et al. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2⑴3;. 34:267.
90.Patti ME., Butte AJ., Grunkhom S., Cusi K., Berria R., Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003. 100:8466–71.
91.Kim JA., Montagnani M., Koh KK., Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006. 113:1888–904.
92.Montagnani M., Quon MJ. Insulin action in vascular endothelium: potential mechanism linking insulin resistance with hypertension. Diabetes Obes Metab. 2000. 2:285–92.
Fig. 1.
General feature of insulin signal transduction pathways. PI 3-kinase branch of insulin signaling regulates GLUT4 translocation and vasodilation in vascular endothelium. MAP-kinase branch of insulin signaling generally regulates growth and mitogenesis and controls secretion of ET-1 in vascular endothelium.91 PI: phosphatidylinositol, GLUT: glucose transporter, MAP: mitogen-activated protein, ET: endothelin.
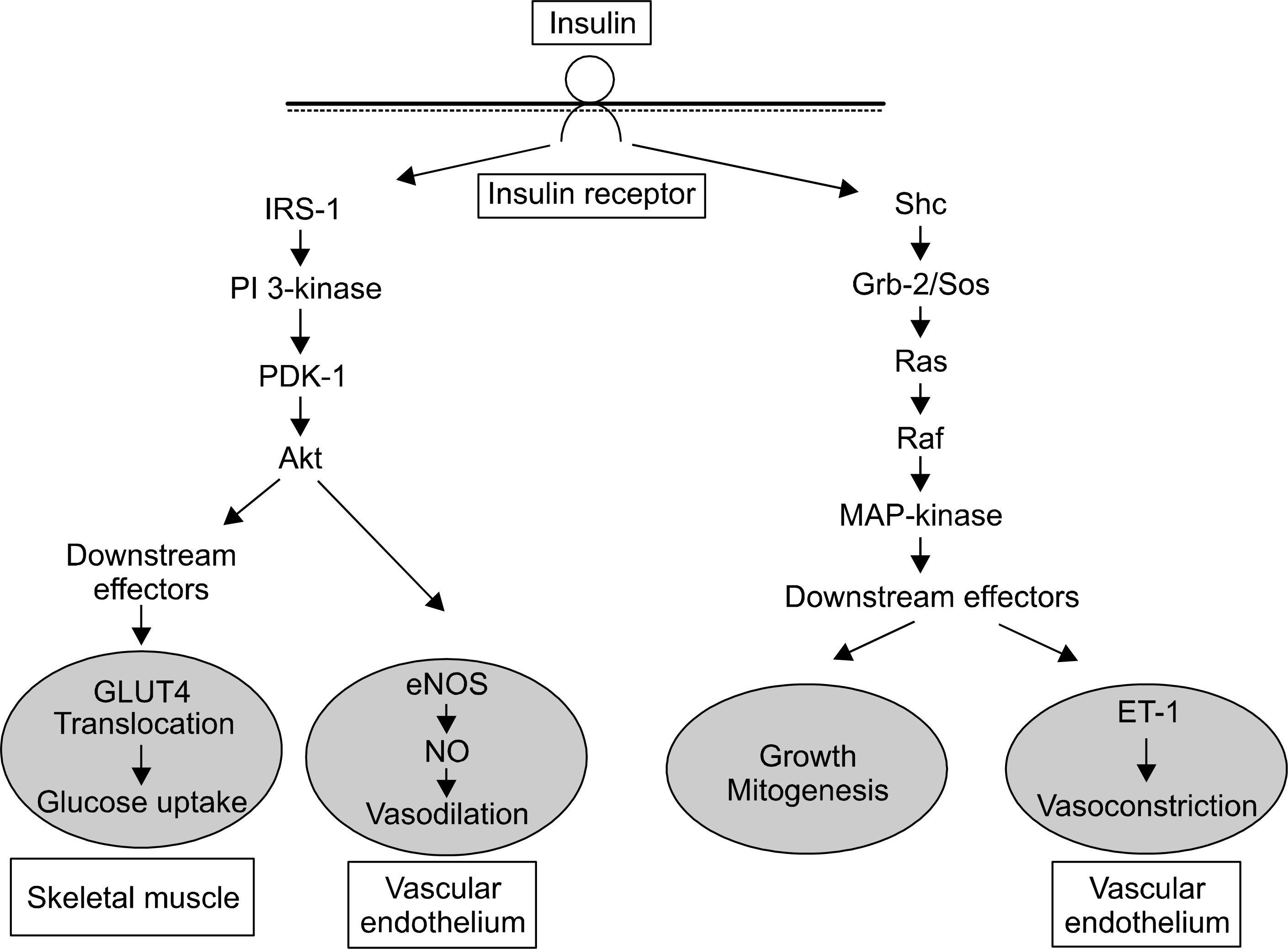
Fig. 2.
Pathway specific insulin resistance creates imbalance between prohypertensive and antihypertensive vascular actions of insulin exacerbated by compensatory hyperinsulinemia.92 SNS: sympathetic nervous system, ET: endothelin, NO: nitric oxide.
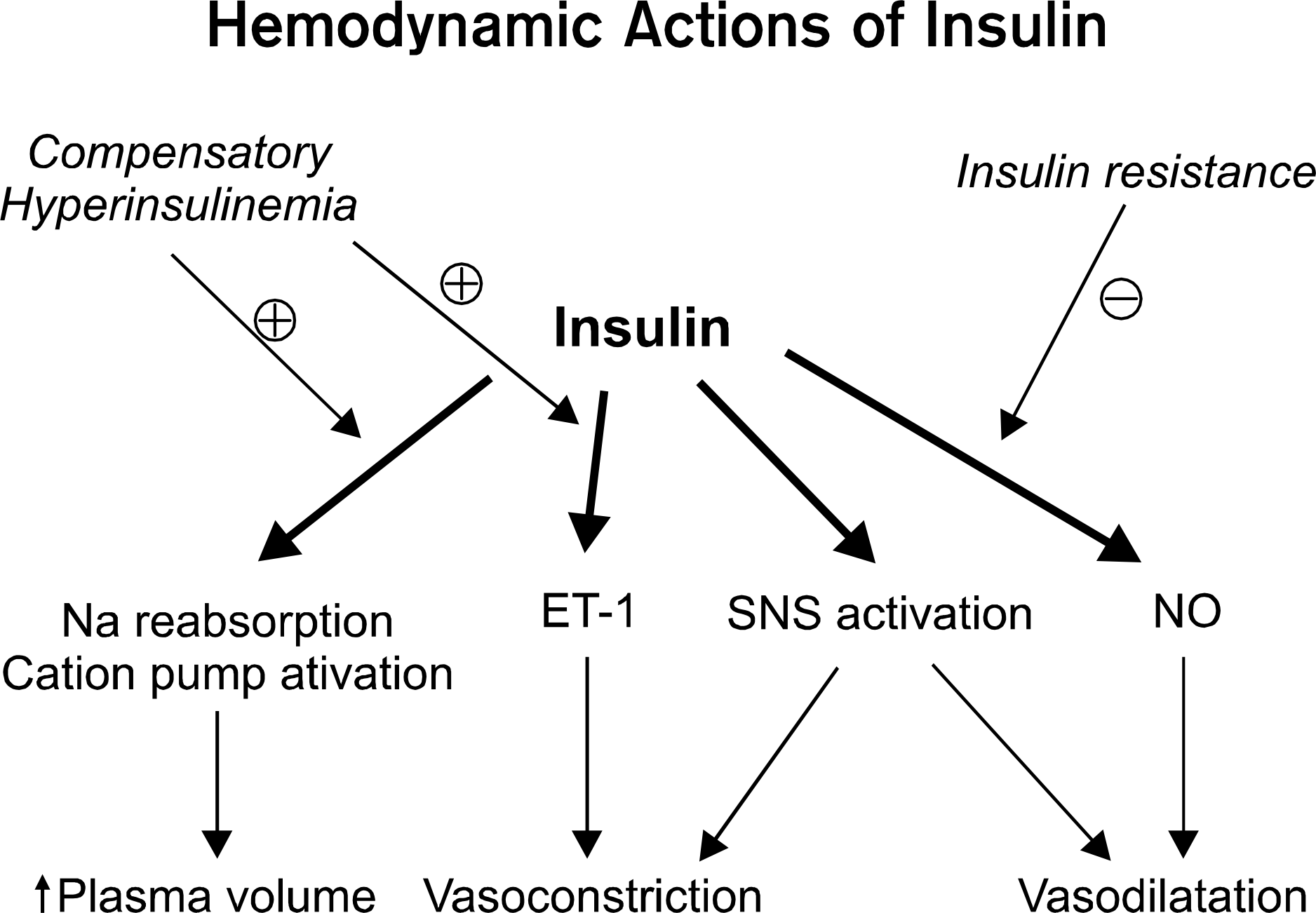
Fig. 3.
Effects of NS-398 (10yM) and U-51605 (ΙΟμΜ) on the insulin-induced relaxations (n=8). NS-398 produced no remarkable changes, but U-51605 almost completely inhibits the insulin- induced relaxation.18 ∗p<0.05.
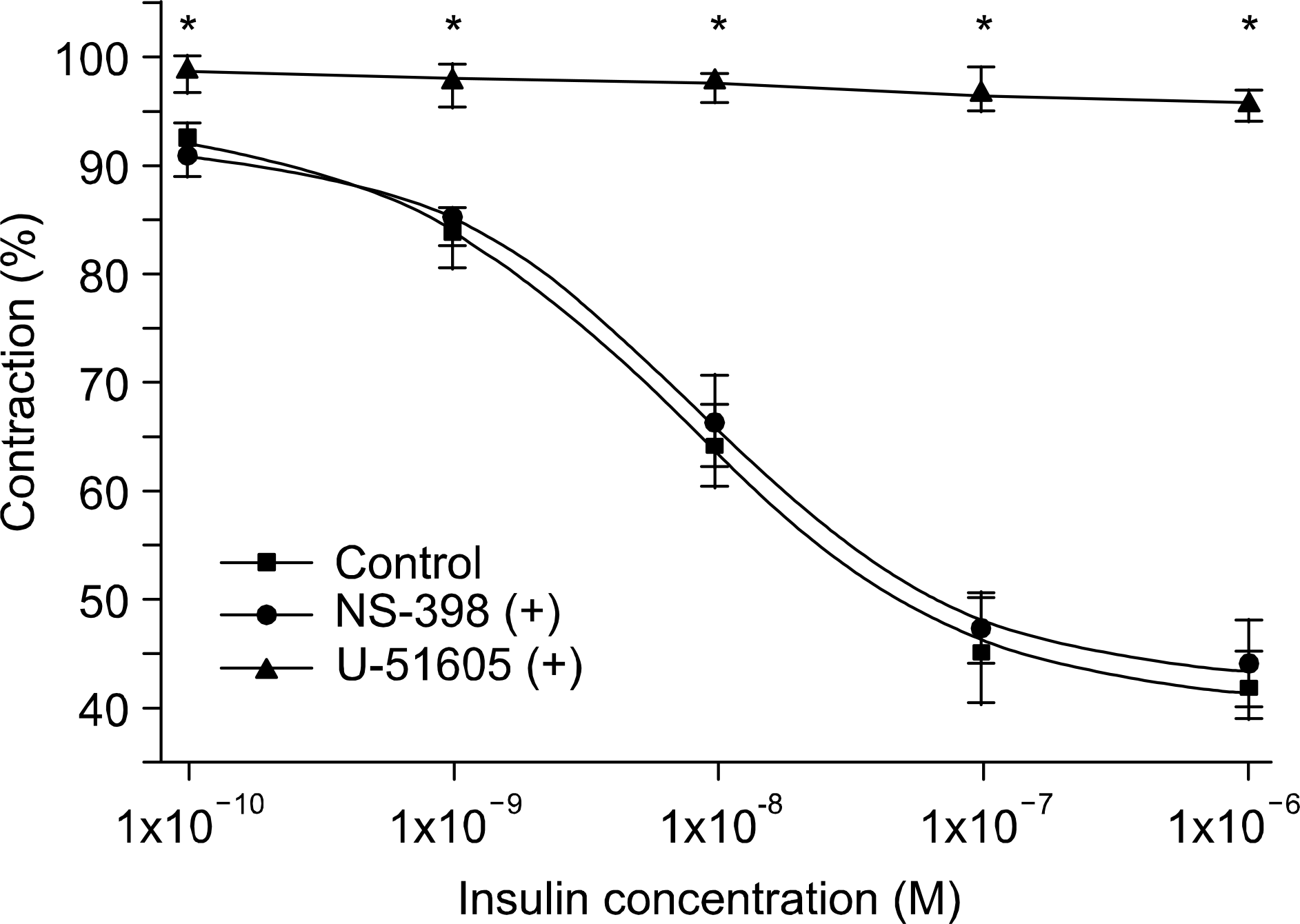
Fig. 4.
ᄂ-type calcium channel activities recorded in whole cell patch clamp mode (n=8). ᄂ-type Ca2+ currents are unaffected by insulin (ΙΟμΜ) treatment but prostacyclin (lOyM) treatment significantly reduces the L-type Ca2+ currents. ∗p<0.05.
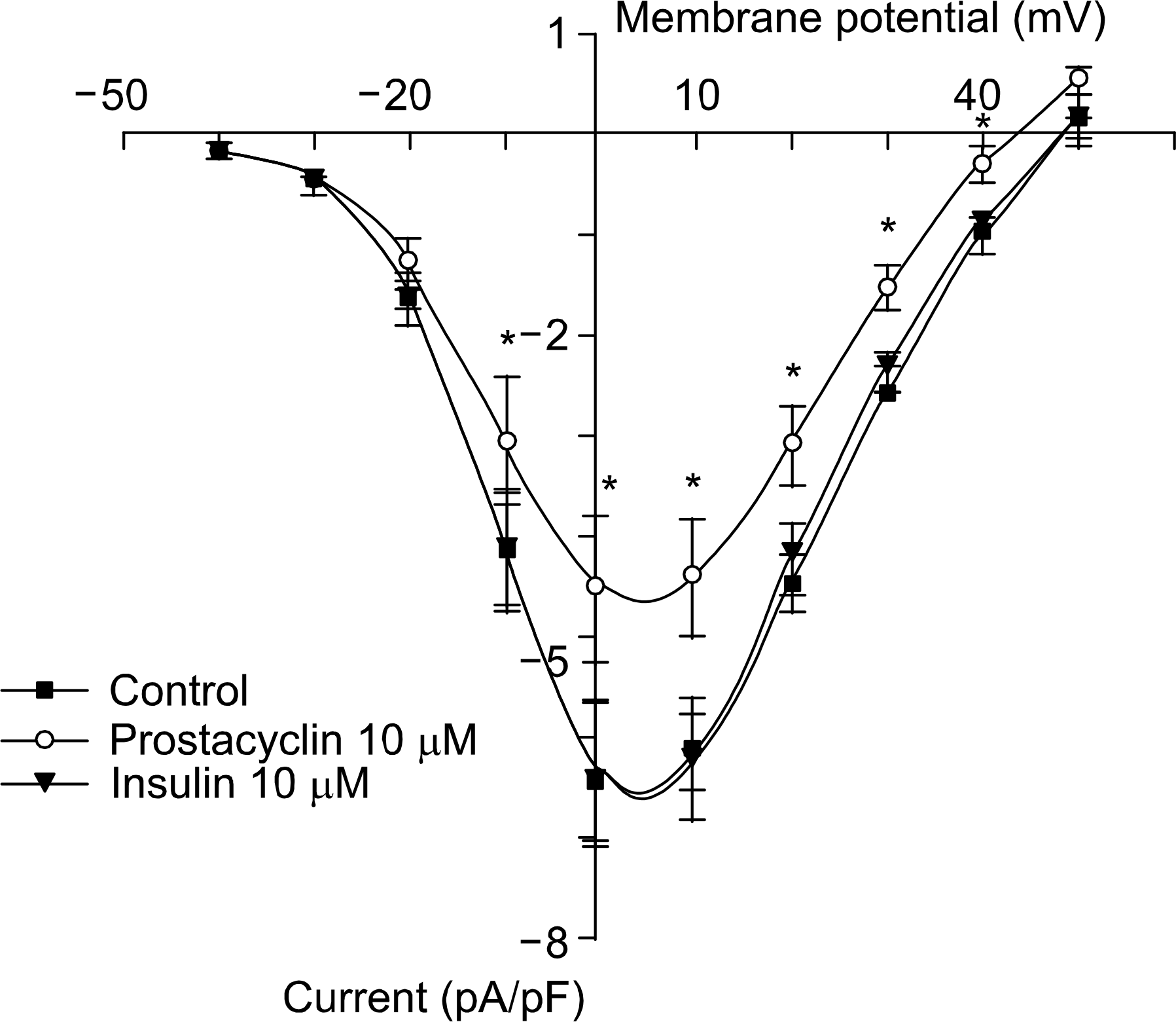
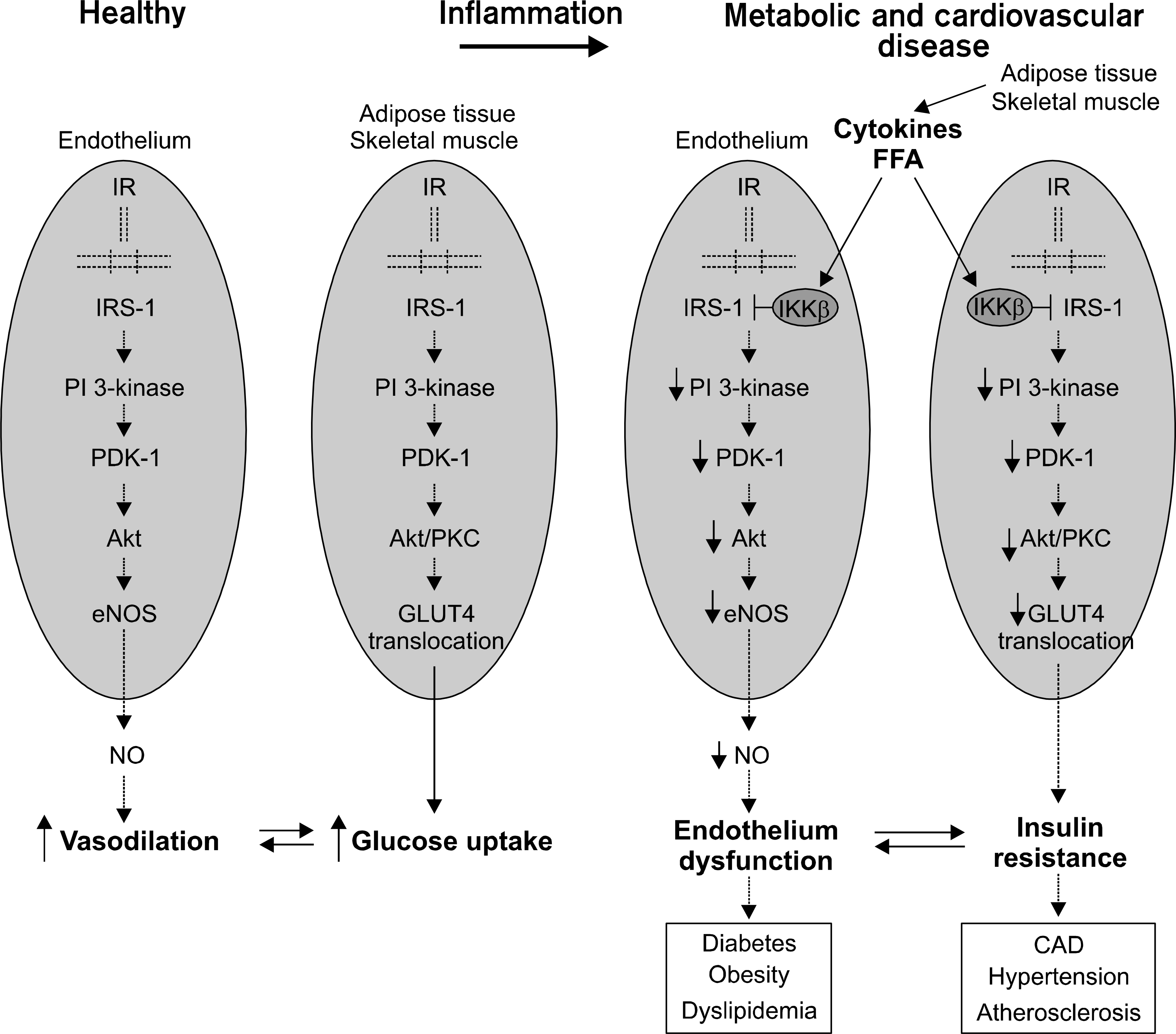
Fig. 5.
Left, Parallel PI 3-kinase-dependent insulin-signaling pathways in metabolic and vascular tissues synergistically couple metabolic and vascular physiology under healthy conditions. IR: insulin receptor. Right, Parallel impairment in insulin-signaling pathways under pathological conditions contributes to synergistic coupling of insulin resistance and endothelial dysfunction. PI: phosphatidylinositol, eNOS: endothelial nitric oxide synthase, PDK: phosphoinositide-dependent kinase, GLUT: glucose transporter.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download