Abstract
Inflammation, an innate immune response mediated by macrophages, forms the first line of defence to protect our body from the invasion of various pathogens. Although inflammation is a defensive response, chronic inflammation has been regarded as the major cause of many types of human diseases such as inflammatory/autoimmune diseases, cancers, neurological diseases, and cardiovascular diseases. Folate receptor (FR) is a cell surface glycosylphosphatidylinositol (GPI)-anchored glycoprotein, and its three isoforms, FR-α, FR-β, and FR-γ, are found in humans. Interestingly, FRs are highly expressed on a variety of cells, including cancer cells and activated macrophages, whereas their expression on normal cells is undetectable, indicating that FR-targeting could be a good selective strategy for the diagnosis and therapeutic treatment of cancers and activated macrophage-mediated inflammatory diseases. Previous studies successfully showed FR-targeted imaging of many types of cancers in animal models as well as human patients. Recently, a number of emerging studies have found that activated macrophages, which are critical players for a variety of inflammatory diseases, highly express FRs, and selective targeting of these FR-positive activated macrophages is a good approach to diagnose and treat inflammatory diseases. In this review, we describe the characteristics and structure of FRs, and further discuss FR-targeted diagnostics and therapeutics of human diseases, in particular, activated macrophage-mediated inflammatory diseases.
Folic acid has emerged as a promising ligand for the selective delivery of imaging and therapeutic agents to target cells, such as cancer cells and activated macrophages, in inflammatory sites (1). As a selective targeting ligand, folic acid has several advantages such as 1) high affinity to its target, folate receptors (FRs), even after conjugation with diagnostic/therapeutic agents, 2) ease of conjugation with a variety of imaging and therapeutic agents, and 3) very low or undetectable expression of its receptors on normal cells, despite its high expression on cancer cells and activated macrophages (1). Folic acid primarily enters non-pathogenic, normal cells through the reduced folate carrier to effect its functions (2), but folic acid linked with conjugating agents only enters cells through the FR (23). FR is a cell surface glycosylphosphatidylinositol (GPI)-anchored glycoprotein, and there are three isoforms in humans: FR-α, -β, and -γ (45). FR-α is overexpressed on many types of cancer cells, including ovary, lung, breast, kidney, brain, endometrium, and colon cancer, whereas FR-β is overexpressed on activated macrophages which are implicated in inflammatory pathologies, including rheumatoid arthritis, psoriasis, Crohn's disease, and systemic lupus erythematosus (6). For this reason, FRs have been regarded as promising molecular targets for both diagnostics and therapeutic treatment of a variety of human diseases. In this review, we will introduce the characteristics and structures of FRs and discuss their expression profiles on normal, as well as pathogenic, cells. Furthermore, we will give an overview of recent studies describing strategies to target FR-β selectively expressed on activated macrophages for the diagnosis and therapy of human inflammatory diseases.
Folic acid, also known as folate and vitamin B9, is essential for cells to generate DNA, RNA, and metabolic amino acids that are required for their proliferation and division (78). Because eukaryotic cells are incapable of synthesizing folic acid, it is delivered into cells through either the reduced folate carrier, which is present in all cell types, or the FR, which is expressed in limited cells (9). Although folic acid is transported into the cells through either system, folate conjugates designed for diagnostics and therapeutics (Fig. 1A) are transported only through FRs by FR-mediated endocytosis (Fig. 1B) (9).
The FR is a cell surface glycoprotein of molecular weight in the range of 38~45 kDa with a high affinity for folic acid (KD<10–9~10–10), and is attached to the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor (45). Human FR (hFR) is encoded by a family of hFR genes, and three isoforms, hFR-α, -β, and -γ, have been reported to date (4). The gene which is predicted to encode hFR-δ was also found from genome data mining; however hFR-δ-expressing cells and tissues have not yet been identified (10).
hFR-α and -β are membrane-associated proteins, whereas hFR-γ is a secreted protein because it does not have the signal peptide for the GPI anchor at its C-terminus (1112). hFRs share 68~79% amino acid sequence identity and have N-glycosylation sites that are critical for their proper folding (1213). hFR-α and -β transport folic acid into cells via receptor-mediated endocytosis. Although all hFRs have been reported to have high binding affinity with folic acid, relative affinities of hFR-α and -β for folate conjugates are significantly different, in the range of 2~100 fold (14). Studies of chimeric hFR constructs showed that amino acid sequences, such as Leu49 in hFR-β, and Ala49, Val104, and Glu166 in hFR-α, are critical for the differential ligand specificities of hFR-α and -β (1516).
FRs are expressed on the cells of different tissues depending on the types of FR isoforms. FR-α is expressed in the epithelial cells of normal tissues, such as type I and II alveolar cells in the lungs, choroid plexus, ovary, fallopian tube, uterus, epididymis, submandibular salivary and bronchial glands, placental trophoblasts, and the basolateral membrane of retinal pigment epithelial cells (51718). Because the FR-α expressed in these normal tissues is distributed on the luminal surfaces of the tissues, it is protected from FR-targeted folate conjugates administered intravenously. However, FR-α is expressed in proximal kidney tubules, which are exposed to folate conjugates filtered from the blood stream. Interestingly, FR-α is overexpressed on many malignant tumor cells of epithelial origin, including lung, ovarian, cervical, endometrial, brain, and breast cancers (45), and significant correlation has been observed between the FR-α expression level and the grade of the tumor (1920).
Although many previous studies have focused on the expression of FR-α on the cells of neoplastic tissues, recent studies have shown that FR-β is expressed on different types of cells. FR-β is a marker protein in normal hematopoiesis of myelomonocytic lineage cells (5), and is expressed in neutrophils, CD34+ hematopoietic progenitor cells, placenta, spleen, and thymus (212223). Expression of FR-β is also found in pathogenic cells, such as acute myelogenous leukemia (AML) cells and chronic myelogenous leukemia (CML) cells (2124). Interestingly, FR-β is highly expressed on activated, but not normal and resting macrophages, which are implicated in the pathogenesis of human inflammatory diseases, such as rheumatoid arthritis, psoriasis, Crohn's disease, systemic lupus erythematosus, atherosclerosis, diabetes, ulcerative colitis, osteoarthritis, glomerulonephritis, and sarcoidosis (6). The expression of FR-β, indeed, was confirmed in synovial macrophages obtained from patients suffering from rheumatoid arthritis and in a mouse rheumatoid arthritis model (2526).
FR-γ is expressed in normal and malignant hematopoietic cells in the spleen, bone marrow, and thymus as well as ovarian, cervical, and uterine cancer cells (1113). Although FR-γ mRNA was detected in lymphoid leukemia cells, a secreted form of FR-γ protein has not been detected in the serum of patients with lymphoid leukemia (4).
Because activated macrophages, which are regarded as key players in the pathogenesis of inflammatory diseases, express a high level of FR-β on their surface, folate-conjugated imaging agents have been designed and synthesized to detect pathogenic activated macrophages in the lesions of inflammatory diseases by selectively targeting FR-β. Folic acid has been linked to a variety of dyes or radiopharmaceuticals, such as FITC, Texas Red, Alexa Fluor, Oregon Green, 99mTechnetium (99mTc), 67Gallium (67Ga), and 111Indium (111In) (272829), and these folate imaging agents have been used for the detection of FR-positive cancer cells and pathogenic activated macrophages.
Because these folate-conjugated imaging agents successfully imaged cancer cells in vitro, in vivo, and even clinically, they were further used for the imaging of activated macrophages in the lesions of inflammatory diseases for their diagnosis. Imaging studies of inflammatory diseases were initially conducted on rheumatoid arthritis to examine whether folate-conjugated imaging agents could selectively detect inflamed arthritic joints. First, arthritic dogs were treated with a folate radiotracer, 99mTc-EC20 (Fig. 1C), to image expected sites of inflammation, and dramatic uptake of 99mTc-EC20 into arthritic joints was observed, whereas normal control dogs displayed background levels of radioactivity (30). Subsequent imaging studies were conducted in adjuvant-induced arthritic rats, and 99mTc-EC20 was used for imaging inflamed arthritic tissues. The resulting images revealed accumulation of 99mTc-EC20 in inflamed joints, livers, and spleens of arthritic rats, but not in those of healthy control rats (26). Importantly, treatment of the arthritic rats with excess free folic acid completely blocked the uptake of 99mTc-EC20 into their joints and organs, strongly indicating that uptake of 99mTc-EC20 is dominantly FR-mediated (26). Depletion of macrophages from arthritic rats with liposomal clodronate treatment also blocked the uptake of 99mTc-EC20, and the binding of folate-conjugated agents was observed only in macrophages in the total cell populations obtained from the livers of atherosclerotic mice (31). An autoradiography study revealed that 99mTc-EC20 was much more accumulated in the atherosclerotic lesions of ApoE-/- mice fed on a high fat diet than their counterparts fed on a normal diet (31). Folate-conjugated fluorescent dyes and radioactive agents specifically targeted asthmatic lung macrophages in a murine asthma model, whereas little uptake by macrophages presented in healthy lung tissue (32). These observations reveal that only FR-expressing macrophages are selective targets for folate-conjugated imaging agents in animal models of human inflammatory diseases. FR-targeted imaging using folate radiopharmaceuticals was also conducted in human patients suffering from both cancer and inflammatory disease. A patent with ovarian cancer was administered folate-diethylenetriaminepentaacetic acid (DTPA)-111In to detect cancer tissues, and uptake was observed within the ovarian cancer tissues (33). Uptake of folate-DTPA-111In and 99mTc-EC20 was also detected in inflamed knee joints of a patient suffering from osteoarthritis and rheumatoid arthritis, whereas no uptake was observed in healthy knees (13435). These FR-targeted in vivo imaging studies of macrophage-mediated inflammatory diseases in animal models and human patients are summarized in Table I, and all data strongly support the idea that folate-conjugated imaging agents can selectively target FR-positive activated macrophages in inflamed tissues. Furthermore, this FR-targeted imaging of activated macrophages could also be applied to other macrophage-mediated inflammatory diseases.
Based on successful FR-targeted imaging of cancers and activated macrophages in inflamed tissues using folate-conjugated imaging agents, folate-drug conjugates were designed and used to treat cancers and inflammatory diseases. Initially, conjugation of conventional chemotherapeutic agents to folic acid was regarded as a good strategy for FR-targeted therapy of human diseases. However, many chemotherapeutic agents are hydrophobic, and hydrophobic chemotherapeutic conjugates exert considerably less target cell specificity than watersoluble ones, therefore, hydrophilic linkers are required to connect folic acid and its chemotherapeutic cargo (36). In addition, it is critical to conjugate the therapeutic agents to folic acid without decreasing the affinity of folic acid for its folate receptor. Most importantly, designing linkers that release free drug inside the cells after FR-mediated endocytosis is a major challenge limiting the therapeutic efficacy of folate-drug conjugates (36). With these guidelines, it is clear that an alternative strategy must be developed to improve the potential of FR-targeted therapy of diseases. Consequently, a new strategy named “FR-targeted immunotherapy,” which minimizes the limitations mentioned above, was developed to treat human diseases (Fig. 2). A highly immunogenic, low molecular weight hapten linked to folate beautifully decorates FR positive cancer cells or activated macrophages, leading to rapid elimination of these targeted cells by macrophages, NK cells, and complement components in the body's immune system through antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cellular cytotoxicity (CDC) (Fig. 2).
Given the therapeutic efficacy of FR-targeted immunotherapy against cancers (37383940), immunotherapy using folate-hapten conjugates was also applied for the targeting of activated macrophages to treat several animal models of inflammatory diseases. Adjuvant- or collagen-induced arthritic rodents, previously immunized with a hapten (FITC), were treated with FR-targeted immunotherapy, resulting in the alleviation of arthritic symptoms by eliminating FR-positive activated macrophages in the inflamed lesions of the rodents (41). FR-targeted immunotherapy also showed therapeutic effects on systemic lupus erythematosus in a murine disease model by alleviating disease symptoms and extending the life span of treated animals (42). Various folate-hapten conjugates were further used for FR-targeted immunotherapy of inflammatory diseases. Initial results with a folate-FITC conjugate showed a good therapeutic effect in animal models of rheumatoid arthritis and systemic lupus erythematosus (4142). Folate conjugates of dinitrophenyl (DNP) and trinitrophenyl (TNP) were used for FR-targeted immunotherapy of rheumatoid arthritis in a rodent animal model, and these folate-hapten conjugates exerted therapeutic effects (43). FR-targeted recombinant immunotoxin was also used for FR-targeted immunotherapy of atherosclerosis. Administration of atherosclerotic ApoE-/- mice with recombinant immunotoxin targeted FR-positive activated macrophages and alleviated the symptoms of atherosclerosis in the animal model (44). One concern that could be raised is whether FR-targeted immunotherapy eliminates the macrophage population in our body during treatment, resulting in significant loss of the body's immunity against pathogen infection. However, the conjugates used for the studies of FR-targeted immunotherapy mentioned above were well-tolerated by all animals, and they did not show any symptoms of pathogen infection or side effects during the immunotherapy. This strongly suggests that the possibility of the destruction of the body's immune system during FR-targeted immunotherapy is negligible, and FR-targeted immunotherapy effectively removes only FR-positive activated macrophages that play a critical role in the pathogenesis of inflammatory diseases.
Given that therapeutic FR-targeted immunotherapy was successful for the treatment of macrophage-mediated inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, and atherosclerosis (Table I), this strategy is promising and could be further applied for the treatment of other types of macrophage-mediated inflammatory diseases.
Inflammation is essential for host defense against pathogen invasion; however, uncontrolled or repeated chronic inflammation leads to pathogenic conditions and diseases such as inflammatory diseases and cancers (4546474849). Macrophages are critical immune cells to initiate inflammatory responses (50), and increasing numbers of studies have successfully proven that activated macrophages, which express high levels of FR-β, are found in the inflamed tissues of a variety of inflammatory diseases and are actively involved in the pathogenesis of these diseases. This strongly suggests that selective targeting of FRs using folate conjugated imaging or therapeutic agents could be a good strategy for the diagnosis, as well as therapeutic treatment, of macrophage-mediated inflammatory diseases. In spite of these successful studies proving the utility of FRs as diagnostic and therapeutic targets for inflammatory diseases, many inflammatory and other human diseases remain to be investigated by FR targeting. Moreover, few therapeutic drugs developed based on the strategy of selective FR targeting have been reported so far for these diseases, which raises the necessity of developing new potential drugs targeting FRs with strong efficacies and minimal toxicities. In conclusion, selective targeting of FRs, especially FR-β on activated macrophages, could be a promising strategy for the diagnosis and treatment of macrophage-mediated inflammatory diseases, and there will be a high demand for the development of FR-targeted efficacious and safe therapeutics.
Figures and Tables
Figure 1
(A) Structure of a folate conjugate. Folate conjugates consist of three major parts: folic acid, which binds with the folate receptor (FR) on target cells, a linker, which connects folate and the imaging/drug agent, and the imaging/drug agent that is necessary for diagnosis and therapy of folate receptor-positive cells in human diseases. (B) FR-mediated endocytosis of folate conjugate. Folate conjugates bind with FRs expressed on various types of cancer cells and activated macrophages, and enter the cells by receptor-mediated endocytosis. Endocytosed folate conjugates in endosomes are then fused with lysosomes inside the cells, and the conjugated agent is released from folate into the cells. After releasing folic acid and the conjugated agent, folate receptors are transported to the cell membrane and recycled to bind with further folate conjugate. (C) Structure of 99mTc-EC20. 99mTc-EC20 consists of two parts: an FR binding moiety (folic acid) and a chelating moiety that forms a pocket to chelate 99mTc.
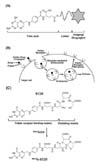
Figure 2
(A) Folate receptor (FR)-targeted immunotherapy by antibody-dependent cellular cytotoxicity (ADCC) by NK cells and macrophages. A folate-hapten conjugate binds with the FR on target cells, and antibodies specific for the hapten directly bind with the hapten on the conjugate. Macrophages and NK cells then recognize the antibody through their Fc receptors and kill the target cells by ADCC. (B) FR-targeted immunotherapy by complement-dependent cellular cytotoxicity (CDC). A folate-hapten conjugate binds with the FR on target cells and antibodies specific for the hapten bind directly to it. Complement components then bind to the antibody, and this binding activates complement cascades, followed by the formation of a membrane attack complex (MAC) at the surface of the target cells, which leads to cell death.
Table I
FR-targeted in vivo imaging and immunotherapy of diseases

| Imaging/Immunotherapy | Diseases | Animal models/Human | References |
|---|---|---|---|
| Imaging | Rheumatoid arthritis | Rat | (26) |
| Dog | (30) | ||
| Human | (34) | ||
| Osteoarthritis | Human | (1, 35) | |
| Atherosclerosis | Mice | (31) | |
| Asthma | Mice | (32) | |
| Ovarian cancer | Human | (33) | |
| Immunotherapy | Lung cancer | Mice | (37, 38) |
| Ovarian cancer | Mice | (39) | |
| Colon cancer | Mice | (40) | |
| Rheumatoid arthritis | Rat/Mice | (41, 43) | |
| Systemic lupus erythematosus | Mice | (42) | |
| Atherosclerosis | Mice | (44) |
References
1. Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008; 41:120–129.

2. Lu JY, Lowe DA, Kennedy MD, Low PS. Folate-targeted enzyme prodrug cancer therapy utilizing penicillin-V amidase and a doxorubicin prodrug. J Drug Target. 1999; 7:43–53.

4. Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007; 26:141–152.

5. Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004; 56:1067–1084.

6. Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv Drug Deliv Rev. 2004; 56:1055–1058.

7. Kamen B. Folate and antifolate pharmacology. Semin Oncol. 1997; 24:S18.
9. Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998; 15:587–627.

10. Spiegelstein O, Eudy JD, Finnell RH. Identification of two putative novel folate receptor genes in humans and mouse. Gene. 2000; 258:117–125.

11. Shen F, Wu M, Ross JF, Miller D, Ratnam M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: protein characterization and cell type specificity. Biochemistry. 1995; 34:5660–5665.

12. Lacey SW, Sanders JM, Rothberg KG, Anderson RG, Kamen BA. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989; 84:715–720.

13. Shen F, Ross JF, Wang X, Ratnam M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry. 1994; 33:1209–1215.

14. Wang X, Shen F, Freisheim JH, Gentry LE, Ratnam M. Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates. Biochem Pharmacol. 1992; 44:1898–1901.

15. Maziarz KM, Monaco HL, Shen F, Ratnam M. Complete mapping of divergent amino acids responsible for differential ligand binding of folate receptors alpha and beta. J Biol Chem. 1999; 274:11086–11091.

16. Shen F, Zheng X, Wang J, Ratnam M. Identification of amino acid residues that determine the differential ligand specificities of folate receptors alpha and beta. Biochemistry. 1997; 36:6157–6163.

17. Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992; 52:3396–3401.
18. Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res. 1992; 52:6708–6711.
19. Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997; 74:193–198.

20. Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev. 1999; 8:775–782.
21. Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, Ratnam M. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999; 85:348–357.

22. Ratnam M, Marquardt H, Duhring JL, Freisheim JH. Homologous membrane folate binding proteins in human placenta: cloning and sequence of a cDNA. Biochemistry. 1989; 28:8249–8254.

23. Reddy JA, Haneline LS, Srour EF, Antony AC, Clapp DW, Low PS. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999; 93:3940–3948.

24. Pan XQ, Zheng X, Shi G, Wang H, Ratnam M, Lee RJ. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood. 2002; 100:594–602.

25. Nakashima-Matsushita N, Homma T, Yu S, Matsuda T, Sunahara N, Nakamura T, Tsukano M, Ratnam M, Matsuyama T. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999; 42:1609–1616.

26. Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, Low PS. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002; 46:1947–1955.

27. Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang ZF, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl Med Biol. 2003; 30:725–731.

28. Siegel BA, Dehdashti F, Mutch DG, Podoloff DA, Wendt R, Sutton GP, Burt RW, Ellis PR, Mathias CJ, Green MA, Gershenson DM. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J Nucl Med. 2003; 44:700–707.
29. Reddy JA, Xu LC, Parker N, Vetzel M, Leamon CP. Preclinical evaluation of (99m)Tc-EC20 for imaging folate receptor-positive tumors. J Nucl Med. 2004; 45:857–866.
30. Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004; 56:1205–1217.

31. Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein e knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med. 2010; 51:768–774.

32. Shen J, Chelvam V, Cresswell G, Low PS. Use of folate-conjugated imaging agents to target alternatively activated macrophages in a murine model of asthma. Mol Pharm. 2013; 10:1918–1927.

33. Mathias CJ, Wang S, Waters DJ, Turek JJ, Low PS, Green MA. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J Nucl Med. 1998; 39:1579–1585.
34. Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009; 113:438–446.

35. Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016; 24:1613–1621.

36. Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005; 94:2135–2146.

37. Lu Y, Sega E, Low PS. Folate receptor-targeted immunotherapy: induction of humoral and cellular immunity against hapten-decorated cancer cells. Int J Cancer. 2005; 116:710–719.

38. Lu Y, You F, Vlahov I, Westrick E, Fan M, Low PS, Leamon CP. Folate-targeted dinitrophenyl hapten immunotherapy: effect of linker chemistry on antitumor activity and allergic potential. Mol Pharm. 2007; 4:695–706.

39. Wen Y, Graybill WS, Previs RA, Hu W, Ivan C, Mangala LS, Zand B, Nick AM, Jennings NB, Dalton HJ, Sehgal V, Ram P, Lee JS, Vivas-Mejia PE, Coleman RL, Sood AK. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015; 21:448–459.

40. Liang X, Luo M, Wei XW, Ma CC, Yang YH, Shao B, Liu YT, Liu T, Ren J, Liu L, He ZY, Wei YQ. A folate receptor-targeted lipoplex delivering interleukin-15 gene for colon cancer immunotherapy. Oncotarget. 2016; DOI: 10.18632/oncotarget.10537.

41. Paulos CM, Varghese B, Widmer WR, Breur GJ, Vlashi E, Low PS. Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis. Arthritis Res Ther. 2006; 8:R77.
42. Varghese B, Haase N, Low PS. Depletion of folate-receptor-positive macrophages leads to alleviation of symptoms and prolonged survival in two murine models of systemic lupus erythematosus. Mol Pharm. 2007; 4:679–685.

43. Yi YS, Ayala-Lopez W, Kularatne SA, Low PS. Folate-targeted hapten immunotherapy of adjuvant-induced arthritis: comparison of hapten potencies. Mol Pharm. 2009; 6:1228–1236.

44. Furusho Y, Miyata M, Matsuyama T, Nagai T, Li H, Akasaki Y, Hamada N, Miyauchi T, Ikeda Y, Shirasawa T, Ide K, Tei C. Novel therapy for atherosclerosis using recombinant immunotoxin against folate receptor beta-expressing macrophages. J Am Heart Assoc. 2012; 1:e003079.
45. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007; 147:227–235.

46. Park YJ, Chung MK, Hwang D, Kim WU. Proteomics in rheumatoid arthritis research. Immune Netw. 2015; 15:177–185.

47. Yan S, Yim LY, Lu L, Lau CS, Chan VS. MicroRNA regulation in systemic lupus erythematosus pathogenesis. Immune Netw. 2014; 14:138–148.

48. Bae S, Kim H, Yu YS, Lee NE, Kong JM, Kim HR, Hwang YI, Song YW, Kang JS, Lee WJ. Identification of CM1 as a pathogenic factor in inflammatory diseases and cancer. Immune Netw. 2011; 11:175–181.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download