Abstract
The intestinal immune system has an ability to distinguish between the microbiota and pathogenic bacteria, and then activate pro-inflammatory pathways against pathogens for host defense while remaining unresponsive to the microbiota and dietary antigens. In the intestine, abnormal activation of innate immunity causes development of several inflammatory disorders such as inflammatory bowel diseases (IBD). Thus, activity of innate immunity is finely regulated in the intestine. To date, multiple innate immune cells have been shown to maintain gut homeostasis by preventing inadequate adaptive immune responses in the murine intestine. Additionally, several innate immune subsets, which promote Th1 and Th17 responses and are implicated in the pathogenesis of IBD, have recently been identified in the human intestinal mucosa. The demonstration of both murine and human intestinal innate immune subsets contributing to regulation of adaptive immunity emphasizes the conserved innate immune functions across species and might promote development of the intestinal innate immunity-based clinical therapy.
The intestine is a unique tissue where a refined balance is maintained between immune responses and tolerance against a variety of environmental factors including microflora and food antigens. In the intestine, aberrant inflammatory responses can cause development of inflammatory bowel disease (IBD), which is characterized by two main clinical forms, Crohn's disease (CD) and ulcerative colitis (UC). Moreover, it was commonly assumed that intestinal inflammation was caused by a disruption in the balance between Th1 and Th2 cytokine responses. In addition, abnormal activation of Th17 cells has been implicated in the pathogenesis of CD. The number of Th1 cells and Th17 cells increased in the lamina propria of patients with IBD compared with healthy individuals, suggesting that a Th1/Th17 pathway may contribute to the development of IBD (1).
At steady state, the number and activity of effector T cells are tightly regulated by Foxp3+ regulatory (Treg) T cells, since excessive Th1 and Th17 responses can result in the gut inflammation. Foxp3+ Treg cell has the ability to suppress inflammatory responses through the production of anti-inflammatory cytokines including IL-10 and transforming growth factor (TGF)-β, in addition to other mechanisms which can inhibit the function of antigen-presenting cells via expression of CTLA-4, LAG-3, CD39 and Nrp-1 (2). The transcription factor Foxp3 has been identified as a master regulator of Treg cells. Indeed, loss of function mutations in Foxp3 causes IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) (3). Intestinal Foxp3+ Treg cells produce a large amount of IL-10 compared with those present in other tissues and play an important role in the maintenance of the gut homeostasis (4). Treg cell-derived IL-10 is thought to regulate the activity of intestinal myeloid cells.
Recently, several innate immune subsets have been reported to modulate the intestinal homeostasis through regulation of adaptive immune responses in mouse (5-7). In the intestine, activity of innate immune myeloid cells such as dendritic cells and macrophages is finely regulated by several mechanisms because the excessive and inadequate initiation of innate immunity leads to the development of IBD. Accordingly, patients with CD possess inappropriate or defective innate immune functions, including cytokine production, pathogen clearance, and recruitment of neutrophils. Genome-wide mapping has identified the genes and genetic loci that contribute to IBD susceptibility, including IL-10, IL-12p40, IL-23R and CARD9 (8,9). Thus, intestinal innate immune cells are responsible for maintenance of the gut homeostasis, and dysregulation of activity of these cells lead to development of IBD through induction of colitogenic effector T cells.
This review focuses on a variety of murine innate immune subsets, which are responsible for maintenance of the gut homeostasis leading to prevention of intestinal inflammation, and human intestinal myeloid cells implicated in pathogenesis of IBD.
The intestinal mononuclear phagocytes could be subdivided several subsets possessing different ability to maintain gut homeostasis either through promoting or preventing T cell responses (10-13), according to expression pattern of characteristic surface marker including CD11b, CD11c, CD103 and CX3CR1. In particular, CD103+ dendritic cells and CX3CR1+ CD11b+ cells have been well characterized in the murine intestine (14-16). Pre-cDCs (cDC precursors) give rise to CD103+dendritic cells whereas Ly6Chigh monocytes differentiate into CX3CR1+ cells. Several studies have revealed that both CD103+ dendritic cells and CX3CR1+ cells are a heterogeneous population implicated in either protective immunity or immune tolerance.
CD103+ dendritic cells can stimulate CD4+ and CD8+ T cell proliferation (17,18) and CD103+ dendritic cells activated by TLR ligands strongly induce cytotoxic T lymphocytes (17). In addition, IL-6-producing CD103+CD11b+ dendritic cells migrate to mesenteric lymph node via expression of CCR7 and induce Th17 development in a transcription factor IRF4-dependent manner (19-21) (Fig. 1A). TLR5-activated CD103+ CD11b+CD11chigh dendritic cells induce Th1/Th17 responses (22). Moreover, recent study revealed that IL-23 production by TLR5+CD103+CD11b+ dendritic cells in response to flagellin promotes epithelial expression of RegIIIγ through induction of IL-22 production by innate lymphoid cells (23) (Fig. 1B). Furthermore, CD103+CD11b+CD24+ dendritic cells have been reported to drive Th17 development by IL-23 production. Thus, CD103+CD11b+ dendritic cells can mediate the maintenance of gut homeostasis through promotion of protective immunity by inducing T cell activation and antimicrobial defense in the intestinal epithelial cells.
At steady state, IL-10-producing CD4+ T cells containing two subsets: Foxp3+ Treg cells and type I regulatory T cells (Tr1 cells). CD103+CD11c+ dendritic cells have been reported to be responsible for immune tolerance to the intestinal antigens by promoting Foxp3+ Treg cell differentiation through the production of retinoic acid and TGF-β (12,24,25) (Fig. 1C). In addition, it has recently been clarified that one of the common probiotics, Bifidobacterium breve can induce Tr1 cell development via TLR2-dependent activation of CD103+ dendritic cells (26). B. breve activates intestinal CD103+ DCs to produce IL-10 and IL-27 via the TLR2/MyD88 pathway thereby inducing IL-10-producing Tr1 cells in the large intestine (Fig. 1D). Oral B. breve administration ameliorated colitis through the induction of intestinal IL-10-producing Tr1 cells (26).
CX3CR1+ mononuclear cells have been identified as major populations in the intestinal lamina propria by using CX3CR1-GFP transgenic mice (27). To date, in the colonic lamina propria, several subsets of CX3CR1+ cell have been characterized, such as CD11c-CX3CR1+, CD11c+CX3CR1+CD68+F4/80+ and CD11c+CX3CR1+CD68-F4/80- cell population (15). Studies have shown that CX3CR1+ cells can drive the induction of Th17 development (28,29). In particular, CX3CR1intermediate CD70+CD11b+ dendritic cells express a series of ATP receptors that can induce Th17 cell development (28) (Fig. 2A). Administration of ATP to germ-free mice with few Th17 cells enhances the accumulation of Th17 cells in the lamina propria, indicating that commensal bacteria-derived ATP is responsible for initiation of Th17 cell development.
CX3CR1+ intestinal innate immune cells do not always induce pro-inflammatory responses, even though a part of these cells can enhance the differentiation of Th17 cells (28,29). IL-10 derived from CX3CR1+ macrophage in a CX3CL1-dependent manner support the expansion of Foxp3+ Treg cells (30). In addition, CX3CR1+ macrophages limit Th17 cells-dependent intestinal inflammation by controlling the bacterial clearance (31). In addition, recent study revealed that antigen cross-presentation by CX3CR1+ cells in the small intestinal lamina propria is responsible for differentiation of IL-10/IL-13/IL-9-expressing CD8+ T cells with ability to suppress antigen-specific activation of CD4+ T cells, leading to inhibition of intestinal inflammation (32).
CX3CR1highCD11b+CD11c+ subset, which has been named regulatory myeloid (Mreg) cells, has been identified to possess a negative regulatory function (33). Mreg cells are distributed in the intestinal lamina propria, suppress CD4+ T cell proliferation in a cell-cell contact-dependent manner, and contribute to the prevention of intestinal inflammation. Mreg cells preferentially associate with CD4+ T cells via highly expressed adhesion molecules such as ICAM-1 and VCAM-1, but do not activate CD4+ T cells owing to the IL-10/Stat3-dependent suppression of CD80/CD86 expression (Fig. 2B). LysMcre; Stat3flox/flox mice, which spontaneously develop colitis, show defective Mreg cell function. Administration of wild-type Mreg cells to Stat3 mutant mice ameliorated intestinal inflammation, indicating that a dysfunction of Mreg cells is involved in the pathogenesis of IBD. Taken together, several subsets of CX3CR1+ innate immune cells contribute to both anti-inflammatory and pro-inflammatory responses in the intestine, suggesting that the disrupted functions of these cells lead to dysregulation of intestinal immune responses resulting in development of IBD.
Intestinal CD11b+CD11c- macrophages produce substantial amounts of IL-10 in response to microflora (34-36) (Fig. 2C). IL-10 produced by intestinal macrophages limits the intestinal inflammation through the persistence of Foxp3 expression in Treg cells (37). In addition, intestinal macrophages-derived IL-10 inhibits IL-12 and TNF-α production against the microbiota in intestinal myeloid cells in an IL-10/Stat3 signal-dependent manner. Consequently, IL-10-deficient mice and myeloid cell-specific Stat3 mutant mice (LysM-cre; Stat3flox/flox) spontaneously develop enteric inflammation.
Apart from CD103+ dendritic cells, CX3CR1+ cells and macrophages, E-cadherin-expressing dendritic cells, which highly express TLRs and produce colitogenic cytokines including IL-6 and IL-23, enhance the intestinal inflammation by increasing Th17 responses (23). Thus, several subsets of intestinal innate immune myeloid cells contribute to maintenance of gut homeostasis, and dysregulation of activities of these cells leads to development of IBD.
Numerous murine studies have shown that intestinal innate immune cells are heterogeneous and comprise several subtypes with different phenotypes and functional properties. Recently, studies have reported human counterpart to murine intestinal macrophages, CD103+ dendritic cells and CX3CR1intermediate CD70+ dendritic cells.
Recent studies have suggested that the intestinal macrophages play important roles in protecting host from invading pathogens and regulating excess immune responses to commensal bacteria by producing IL-10 (Fig. 3A). Interestingly, numbers of CD14+ macrophage are increased in patients with CD and produce greater amounts of colitogenic cytokines including IL-6, IL-23 and TNF-α against microbiota than do healthy individuals (38). Furthermore, human intestinal CD14+ macrophage-derived IL-23 may enhance colitogenic IL-17 and IFN-γ-producing T cell differentiation, suggesting that abnormal innate immune responses by macrophages play a major role in the pathogenesis of IBD (38-41) (Fig. 3B).
Similar to murine CD103+ dendritic cells, human CD103+ dendritic cells that can induce homing receptors such as CCR9 and integrin α4β7 on T cells have been reported (18). In addition, transcription factor IRF4-expressing CD103+CD141- SIRPαhigh dendritic cells have been clarified in the humane small intestine (21) (Fig. 3A), suggesting that these cells might be equivalent to murine CD103+CD11b+ dendritic cells possessing the ability to enhance Th17 cell differentiation.
Several findings on human counterpart to the murine intestinal macrophages and CD103+ dendritic cells have been shown, as described above. Furthermore, it has recently been identified HLA-DRhighCD14+CD163low cells as equivalents of CX3CR1intermediateCD70+CD11b+ dendritic cells driving Th17 cell differentiation (42).
In the human intestinal mucosa, HLA-DRhighLin- cells could be divided into CD14+CD163low, CD14+CD163high, CD14- CD11clow, and CD14-CD11chigh subsets. CD14+CD163low cells can induce Th17 differentiation by high expression of IL-6, IL-23p19, TNF-α and IL-1β, via TLR2, TLR4 and TLR5 signal pathway even in steady state (Fig. 3A). In addition, CD14+ CD163low cells produce a low level of IL-10. Recent study reported that murine CX3CR1intermediate dendritic cells promoting Th17 polarization produce not only IL-6 and TNF-α, but also IL-10. In addition, CD14+CD163low cells express both macrophage-related and dendritic cell-related marker as well as CX3CR1intermediate dendritic cells, suggesting that CD14+CD163low cells are the putative equivalents of CX3CR1intermediat cells. Furthermore, the activity of CD14+CD163low cells to drive Th17 differentiation is increased in the intestinal mucosa of patients with CD (Fig. 3B), further suggesting that CD14+ CD163low cells might play a crucial role in pathogenesis of CD.
Recent advances have revealed crucial roles of innate immune cells for protection against foreign pathogens and immunological tolerance in mice. The aberrant activation of innate immunity in the intestinal mucosa can lead to development of several inflammatory diseases by triggering Th1/Th17 responses. Th1 and Th17 cells contribute to the protective responses to pathogens during physiological conditions in steady state, whereas excessive activation of these cells is responsible for intestinal inflammation. Thus, Foxp3+ Treg cells abundantly exist in the intestinal mucosa and inhibit innate and adaptive immune responses for preventing intestinal inflammation.
To date, several studies have clarified human counterparts to the murine intestinal innate immune subsets inducing Th1/Th17 differentiation. Compared with innate inflammatory subsets, other subsets inducing Foxp3+ Treg cells in the human intestinal mucosa remains poorly characterized. Thus, further studies to identify innate immune subsets driving Foxp3+Treg cells in the human intestine may promote advances in therapeutic approaches for IBD.
A previous study showed that IL-10-producing Foxp3+CD4+ CD25+ cells accumulate at inflamed sites in patients with IBD (43), indicating that IBD is not simply a result of defective Treg cell function. In LysM-cre; Stat3flox/flox mice, the severity of intestinal inflammation was improved by administration of wild-type Mreg cells. These findings raise the possibility that the impaired Mreg function is responsible for onset or/and progression of IBD. Accordingly, it will be important for the clinical application of Mreg cells for intestinal inflammation to determine whether Mreg cells are present in the human intestine and fully functional in patients with IBD.
Figures and Tables
Figure 1
Murine CD103+ dendritic cells. (A) IRF4+CD103+CD11b+ dendritic cells induce Th17 development through the production of IL-6. (B) IL-23-expressing TLR5+CD103+CD11b+ dendritic cells promote differentiation of Th1 and Th17 cells and induce antimicrobial peptide RegIIIg from epithelial cells through the induction of IL-22 by innate lymphoid cells (ILC). (C) CD103+CD11c+ dendritic cells induce differentiation of Foxp3+ Treg cells through the production of retinoic acid and TGF-β. (D) B. breve enhances accumulation of Tr1 cells via TLR2 signaling-dependent activation of CD103+CD11c+ dendritic cells.
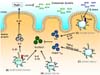
Figure 2
Murine innate myeloid subsets. (A) CX3CR1intermediateCD70+CD11b+ dendritic cells can induce Th17 cell development in a commensal bacteria-derived ATP dependent manner. (B) Mreg cells tightly regulate Th17/Th1 cell proliferation in an IL-10/Stat3-dependent fashion. (C) Macrophages mediate sustained expression of Foxp3 in Treg cells by producing IL-10. In addition, macrophage-derived IL-10 acts on intestinal macrophages, dendritic cells and neutrophils to inhibit colitogenic cytokine production including IL-12p40, TNF-α, IL-23 and IL-6, which contribute to the initiation of Th1/Th17 cell development.
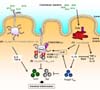
Figure 3
Human intestinal innate myeloid cells. (A) At steady state, CD14+ macrophages play important roles in maintaining immune tolerance by producing IL-10. In contrast, IRF4-expressing CD103+ CD141-SIRPαhigh dendritic cells and CD14+CD163low cells activate pro-inflammatory pathway for host defense through the promotion of Th17 development. (B) In patients with Crohn's disease, IL-23-expressing CD14+ macrophages enhance colitogenic IL-17 and IFN-γ-producing T cell differentiation and the activity of CD14+CD163low cells to drive Th17 differentiation is increased.
References
1. Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 2008; 1:339–349.

2. Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30:636–645.

3. Sakaguchi S. The origin of Foxp3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003; 112:1310–1312.

4. Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999; 190:995–1004.

5. Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010; 140:859–870.

6. Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011; 12:9–23.

7. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011; 474:298–306.

8. Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010; 42:1118–1125.

9. Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011; 43:246–252.

10. Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009; 31:377–388.

11. Varol C, Zigmond E, Jung S. Securing the immune tightrope:mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010; 10:415–426.

12. Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008; 8:435–446.

13. Laffont S, Powrie F. Immunology:Dendritic-cell genealogy. Nature. 2009; 462:732–733.
14. Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009; 31:502–512.

15. Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010; 184:2026–2037.

16. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009; 31:513–525.

17. Fujimoto K, Karuppuchamy T, Takemura N, Shimohigoshi M, Machida T, Haseda Y, Aoshi T, Ishii KJ, Akira S, Uematsu S. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol. 2011; 186:6287–6295.

18. Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008; 205:2139–2149.

19. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009; 206:3101–3114.

20. Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013; 38:958–969.

21. Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013; 38:970–983.

22. Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008; 9:769–776.

23. Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012; 36:276–287.

24. Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005; 202:1063–1073.

25. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007; 204:1775–1785.

26. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012; 8:e1002714.

27. Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005; 307:254–258.

28. Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008; 455:808–812.

29. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007; 8:1086–1094.

30. Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O. Intestinal tolerance requires gut homing and expansion of Foxp3+ regulatory T cells in the lamina propria. Immunity. 2011; 34:237–246.

31. Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011; 121:4787–4795.

32. Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O'Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, Reinecker HC. Circulatory antigen processing by mucosal dendritic cells controls CD8(+). Immunity. 2013; 38:153–165.

33. Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, Yamamoto M, Yagita H, Takeda K. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci U S A. 2012; 109:5010–5015.

34. Ueda Y, Kayama H, Jeon SG, Kusu T, Isaka Y, Rakugi H, Yamamoto M, Takeda K. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010; 22:953–962.

35. Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999; 10:39–49.

36. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993; 75:263–274.

37. Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009; 10:1178–1184.

38. Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008; 118:2269–2280.
39. Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010; 33:279–288.

40. Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Kitazume MT, Takayama T, Okamoto S, Koganei K, Sugita A, Kanai T, Hibi T. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009; 183:1724–1731.

41. Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009; 30:92–107.

42. Ogino T, Nishimura J, Barman S, Kayama H, Uematsu S, Okuzaki D, Osawa H, Haraguchi N, Uemura M, Hata T, Takemasa I, Mizushima T, Yamamoto H, Takeda K, Doki Y, Mori M. Increased Th17-Inducing Activity of CD14+ CD163low Myeloid Cells in Intestinal Lamina Propria of Patients with Crohn's Disease. Gastroenterology. 2013; 145:1380–1391.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download