Abstract
Background
Dendritic cell (DC)-based vaccines are currently being evaluated as a novel strategy for tumor vaccination and immunotherapy. However, inducing long-term regression in established tumor-implanted mice is difficult. Here, we show that deoxypohophyllotoxin (DPT) induces maturation and activation of bone marrow-derived DCs via Toll-like receptor (TLR) 4 activation of MAPK and NF-κB.
Methods
The phenotypic and functional maturation of DPT-treated DCs was assessed by flow cytometric analysis and cytokine production, respectively. DPT-treated DCs was also used for mixed leukocyte reaction to evaluate T cell-priming capacity and for tumor regression against melanoma.
Results
DPT promoted the activation of CD8+ T cells and the Th1 immune response by inducing IL-12 production in DCs. In a B16F10 melanoma-implanted mouse model, we demonstrated that DPT-treated DCs (DPT-DCs) enhance immune priming and regression of an established tumor in vivo. Furthermore, migration of DPT-DCs to the draining lymph nodes was induced via CCR7 upregulation. Mice that received DPT-DCs displayed enhanced antitumor therapeutic efficacy, which was associated with increased IFN-γ production and induction of cytotoxic T lymphocyte activity.
Recently, much research has focused on the biology of dendritic cells (DCs) and their possible clinical use as cellular adjuvants in treating patients with chronic tumors (1). The generation of an optimal immune response often requires the presence of CD4+ T helper cells as well as the expression of T cell-specific antigen on antigen-presenting cells (APCs) (2). As potent APCs, DCs possess immune sentinel properties which enable, the induction of primary immune responses and initiation of T cell responses against microbial pathogens and tumors (3,4). Immature DCs capture and process exogenous agents within the peripheral tissues in which they mature. Maturing DCs then migrate to the lymphoid organs where they stimulate naïve T cells by signaling through the major histocompatibility complex (MHC) and co-stimulatory molecules (5). The maturation and differentiation of DCs require activation via phosphorylation of the mitogen-activated protein kinases (MAPK), including extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, as well as the transcription nuclear factor-κB (NF-κB) (6). DCs can also induce proliferation and generation of specific cytotoxic T lymphocyte (CTL) and helper T cells via stimulation of MHC class I and II antigen presentation (7). Interestingly, nonspecific DC stimulators, including bacterial outer membrane protein A (OmpA), can induce DC-mediated MHC class I-primed specific CTL responses. OmpA-pulsed DCs also induce protective antitumor responses in vivo (8).
In a tumor-bearing host, DCs present tumor peptides in association with MHC class I and II molecules to stimulate specific CD4+ and CD8+ T cell responses (9). Moreover, apoptotic tumor cells and tumor lysates (TP) provide DCs with a comprehensive source of tumor antigens that are used to cross-prime effector T cells (10-12). Formation of TP such as apoptotic tumor bodies in situ is generally thought to result from spontaneous tumor cell death or the activity of cytotoxic effector T cells (13,14). Recently, new approaches to tumor immunotherapy have focused on the enhancement of effector T cell responses by the activation of innate immune cells through their receptors. Among others, receptor agonists (8,15) have been utilized to obtain such results. Pathogen recognition receptors such as the Toll-like receptors (TLR) have been the primary targets of DC activation (16,17). Indeed, they have already been used as immunotherapeutic agents in treating cancer patients (18). TLR agonists stimulate DC inflammatory cytokine production, including interleukin (IL)-12, thereby activating interferon (IFN)-γ-secreting Th1 cells, natural killer cells, and CD8+ CTL (19). Generally, most TLR agonists promote Th1 cells (20). We and others have also demonstrated that certain pathogen-derived molecules that enhance IL-12 and inhibit IL-10 production by DCs also promote the induction of IFN-γ-secreting T cells (21,22).
Deoxypodophyllotoxin (DPT) is an active component and major lignan of the traditional plant Anthriscus sylvestris. DPT also known exhibits exert antitumor (23) and antiviral activities (24). Until now, the cellular targets of DPT in the immune system have remained unknown. We have attempted to characterize the effects of DPT on the maturation and functional properties of murine bone marrow (BM)-derived DCs. Additionally, their functional effects on DC-mediated T cell immunity and antitumor activity were investigated.
Male 6~8 week-old C57BL/6 (H-2Kb and I-Ab) and BALB/c (H-2Kd and I-Ad) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). C.Cg-Tg (DO11.10, H-2d, OT-II) mice carrying the MHC class II-restricted rearranged T cell and C57BL/6-Tg (TcraTcrb, H-2b, OT-1) mice containing transgenic inserts for mouse Tcra-V2 and Tcrb-V5 genes, which encode recognition of OVA257-267 were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were housed in a pathogen-free environment within our animal facility for at least 1 week before use and were used in accordance with the institutional guidelines for animal care. C57BL/6-derived tumor cell lines, F10 sublines of B16 melanoma, were obtained from the Korean Cell Line Bank (Seoul, Korea). B16F10 melanoma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM L-glutamine, 1,000 U/ml Penicillin, 50 µg/ml streptomycin, and 10% fetal bovine serum (FBS). Cells were maintained at 37℃ in 5% CO2. Confluent growth was obtained in 100 mm diameter dishes and cells were routinely passaged every 2 days.
DPT was isolated from the dried roots of Anthriscus sylvestris as described by Jin et al. (25). Briefly, whole compounds from the roots of Anthriscus sylvestris were extracting by ethanol extraction and target compound (DPT, mp 166~167℃, [α]D23-110° (c=1.0, CHCl3)) was extracted by HPLC. The purity of this compound was above 99.5% based on HPLC analysis. The DPT used in this study ran as a single spot on thin layer chromatography and a solution was prepared by dissolving pure DPT in dimethyl sulfoxide (DMSO) diluted with DMEM media. DPT was dissolved in DMSO for further study. The final concentrations of DMSO were adjusted to 0.1% (v/v) in the culture media.
Recombinant mouse (rm) GM-CSF and rmIL-4 were purchased from R&D Systems. Dextran-FITC (molecular mass 40,000), and LPS (from Escherichia coli 055:B5) were obtained from Sigma-Aldrich. An endotoxin filter (END-X) and endotoxin removal resin (END-X B15) were acquired from Associates of Cape Cod. Cytokine ELISA kits for murine IL-12 p70, IL-2, IL-4, IL-10, and IFN-γ were purchased from BD PharMingen. FITC- or PE-conjugated monoclonal antibodies (mAbs) used for flow cytometry to detect CD11c (HL3), CD80 (16-10A1), CD86 (GL1), CD40 (1C10), IAb β-chain (AF-120.1), H2Kb (AF6-88.5), CD4 (L3T4), IL-12 p40/p70 (C15.6), and IL-10 (JESS-16E3), as well as isotype-matched control mAbs and biotinylated anti-CD11c (N418) mAb, were purchased from BD PharMingen. To detect protein levels by western blotting, anti-phospho-ERK, anti-ERK, anti-phospho-p38, and anti-p38 were purchased from Cell Signaling. Anti-p65 Ab was from Abcam.
DCs were generated from murine whole bone marrow (BM) cells. Briefly, BM was flushed from the tibiae and femurs of C57BL/6 and BALB/c mice and depleted red cells with ammonium chloride. The cells were plated in 6-well culture plates (106 cells/ml, 3 ml/well) and cultured at 37℃ in 5% CO2 and OptiMEM (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 5×10-5 M β-mercaptoethanol, 10 mM HEPES (pH 7.4), 20 ng/ml rmGM-CSF, and rmIL-4. On day 3 of culture, floating cells were gently removed and fresh medium was added. On day 6 or 7, nonadherent cells and loosely adherent proliferating DC aggregates were harvested for analysis or stimulation, or in some experiments, replated into 60 mm dishes (106 cells/ml, 5 ml/dish). On day 6, 80% or more of the nonadherent cells expressed CD11c. To obtain highly purified populations for subsequent analyses, the DCs were labeled with bead-conjugated anti-CD11c mAb (Miltenyi Biotec) followed by positive selection on paramagnetic columns (LS columns, Miltenyi Biotec) according to the manufacturer's instructions. The purity of the selected cell fraction was >95%.
DPT and B16F10-tumor lysates (B16-TP) were dissolved in culture media and were added to cultures of isolated DCs in six-well plates (106 cells/ml, 2 ml/well). For the analysis of apoptosis, DCs were stimulated with DPT in media or media alone, and apoptosis was analyzed over time by staining of surface-exposed phosphatidylserine with FITC-annexin V and propidium iodine (BD PharMingen kit).
On day 6, BM-DCs were harvested, washed with PBS, and resuspended in fluorescence activated cell sorter (FACS) washing buffer (2% FBS and 0.1% sodium azide in PBS). The cells were blocked with 10% (v/v) normal goat serum for 15 min at 4℃, and stained with phycoerythrin (PE)-conjugated anti- H-2Kb [major histocompatibility complex (MHC) class I], anti-I-Ab (MHC class II), anti-CD40, anti-TLR4, anti-CD80, and anti-CD86 with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c (PharMingen, San Diego, CA) for 30 min at 4℃. The stained cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
In brief, 2×105 cells were equilibrated at 37℃ or 4℃ for 45 min and pulsed with fluorescein-conjugated dextran (40,000 molecular mass, Sigma-Aldrich) at a concentration of 1 mg/ml. Cold staining buffer was added to stop the reaction. The cells were washed 3 times and stained with PE-conjugated anti-CD11c Abs, and then analyzed with the FACSCalibur. Nonspecific binding of dextran to DCs was determined by incubation of DCs with FITC-conjugated dextran at 4℃ to obtain a background level. The medium used in the cultures with DPT stimulation was supplemented with GM-CSF, which is required for DCs to capture antigen.
Cells were blocked with 10% (v/v) normal goat serum for 15 min at 4℃, and stained with FITC-conjugated CD11c+ antibody for 30 min at 4℃. Cells stained with the appropriate isotype-matched Ig were used as negative controls. The cells were fixed and permeabilized with the Cytofix/Cytoperm kit (PharMingen). Intracellular IL-12p40/p70 and IL-10 were detected with fluorescein R-PE-conjugated antibodies (Phar-Mingen) in permeation buffer. The cells were analyzed on a FACSCalibur flow cytometer with the CellQuest program. The presence of murine IL-12p70, IL-2, IL-4, and IFN-γ was measured using an ELISA kit (R&D Systems).
Responder CD8+ T cells recognizing OVA257-264 were isolated from the spleens of C57BL/6-Tg (TcraTcrb, H-2b, OT-1) mice via a MACS column (Miltenyi Biotec, Gladbach, Germany). CD8+ T cells were labeled with bead-conjugated anti-CD8 mAb (Miltenyi Biotec) followed by positive selection on paramagnetic columns. The lymphocyte population (98% of CD8+ T cells) was then washed twice in PBS and labeled with carboxyfluorescein succinimidyl ester (CFSE), as previously described (26). Stimulator DCs (1×104) derived from C57BL/6 mice were exposed to DPT (100 nM) or LPS (200 ng) for 24 h, washed thoroughly, and co-cultured with 1×105 CFSE-labeled T cells in 96-well U-bottom plates for another 3 days. The cells were harvested and washed in PBS. CFSE dilution was assessed by flow cytometry. A negative control (CD8+ T cells in media alone), a specific antigen control (1 µM OVA257-264) and a positive control (CD8+ T cells in 200 ng/ml LPS) were created for each experiment. CD4+ splenic T cells (2×106/ml) form DO11.10 mice were co-cultured with (2×105/ml) BALB/c DCs in 96-well U-bottom plates for 24 h in the presence or absence of 100 nM DPT. To all experimental groups except the specific antigen control, 1 µM OVA323-339 peptide was added. Cytokine profiles, e.g., IFN-γ (Th1) and IL-4 (Th2) were analyzed by flow cytometry. Supernatants from these cultures were collected after 2 days and analyzed by ELISA.
The cells were exposed to LPS (200 ng) or 100 nM DPT. Following 15, 30, and 45 min incubation at 37℃, cells were washed twice with cold PBS and lysed with modified RIPA buffer (1.0% NP-40, 1.0% sodium deoxycholate, 150 nM NaCl, 10 mM Tris-HCl [pH 7.5], 5.0 mM sodium pyrophosphate, 1.0 mM NaVO4, 5.0 mM NaF, 10 mM/ml leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride) for 15 min at 4℃. Lysates were cleared by centrifuging at 14,000×g for 20 min at 4℃. The protein content of cell lysates was determined using the Micro BCA assay kit (Pierce, Rockford, IL). Equivalent amounts of proteins were separated by 10% or 12% SDS-PAGE and analyzed by western blotting using anti-phospho- ERK1/2 (p-ERK, Cell Signaling, MA) or anti-phospho-p38 (p-p38, Cell Signaling, MA) MAPK mAb for 3 h. Following 3 washes with Tris buffered saline with Tween (TBST), membranes were incubated with secondary horse radish peroxidase (HRP)-conjugated anti-mouse IgG for 2 h. After washing, the blots were developed using the enhanced chemiluminescence (27) system (Amersham). DC nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). NF-κB p-p65 subunits in the nuclear extracts were detected by western blot analysis with an anti- NF-κB p-p65 subunit Ab (p-p65, Abcam, UK).
TLR PCR primers included the following: TLR1, 2, 4, 5, and 6. Quantitative amounts of each gene were standardized against the GAPDH housekeeping gene. Real-time PCR was performed using a BioRad MiniOpticon System (BioRad Laboratories, Ltd) with SYBR green. Reactions were performed in a total volume of 20µl-including 10µl 2x SYBR Green PCR Master Mix (Applied Biosystems), 1µl of each primer at 10µM concentration and 1µl of the reverse-transcribed cDNA template. The cycling protocols was as follows: denaturation (95℃ for 10 min), amplification repeated 40 times (95℃ for 30 s, 52℃ for 30 s, 72℃ for 30 s, and acquisition temperature for 15 s). For each sample, ddCt (crossing point) values were calculated as the Ct of the target gene minus the Ct of the GAPDH gene. Gene expression was derived according to the equation 2-ddCt; changes in gene expression were expressed in relation to the basal level.
CD11c bead-purified DCs were labeled with CFSE, according to the manufacturer's instructions. Thereafter, 1×106 untreated, B16-TP-DCs, DTP-DCs were injected subcutaneously into one hind footpad of normal syngeneic recipients (C57BL/6). Popliteal lymph nodes were removed 24 h after DCs injection, the optimal time described by Abe et al. (28). Ipsilateral inguinal LN were also removed and served as negative controls. LN cells were analyzed by flow cytometry. The number of DCs migrating to draining (D) LN was determined as follows; percent green fluorescent cells in DLN×total number of cells recovered from DLN/number of DCs injected.
B16-TP were prepared as previously described (14). B16F10 melanoma cells were resuspended in extraction buffer containing 0.01 M/L Tris-HCl (pH 7.2) and 0.2 mM/L CaCl2 (10 ml per gram of tumor cells) and homogenized for 3 min on ice using a Silverson homogenizer. Cell extracts were harvested by centrifugation at 1,000×g for 5 min to remove cellular debris. For tumor lysate pulsing 5 to 10×106 DCs were pulsed with B16-TP at a ratio 3:1 (three tumor cell lysates per dendritic cells) in OptiMEM (Invitrogen Life Technologies) supplemented with 10% FBS, 20 ng/ml rmGM-CSF, and rmIL-4 at 37℃ overnight.
C57BL/6 mice (ten each group) were subcutaneously. injected in their right flank with 1×106 viable B16F10 tumor cells. Mice were monitored daily for tumor progression or regression. Mice were subcutaneously vaccinated with 1×106 DCs with medium, B16-TP pulsing, and DPT treated DCs, respectively. The tumor length (L) and width (W) was measured at different time points and tumor volume was determined by L×W2/2.
Splenic lymphocytes were isolated form killed tumor implanted mice 5 days after the last injection in each DC vaccination groups. The lymphocytes were co-cultured with inactivated B16F10 cells (treated by 100 µg/ml) mitomycin for 30 min for 7 day in the presence of recombinant IL-2 (20 U/ml) and then collected as CTL effector cells. CTL activity was determined by lactate dehydrogenase (LDH) release assay with a CytoTox96 Non-Radioactive Cytotoxicity Assay Kit (Promega). The target cells (B16F10) were washed 3 times with RPMI-1640 containing 10% FBS to remove LDH derived from lysed cells. The amount of released LDH was detected in an ELISA reader at a wavelength of 490 nm.
Non-adherent splenocytes derived from tumor implanted mice 7 days after their final injection were washed with twice with PBS. The splenic lymphocytes (2×106 cells/ml) were stimulated with inactivated B16F10 cells at a 10:1 ratio. Supernatants were collected for IL-2 and IFN-γ assays. Cytokines were quantified with an ELISA kit (R&D systems).
Results are presented as the means±standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan's post hoc test using SPSS version 11.0 (SPSS, Inc., Chicago, IL). Throughout the figures and legends the following terminology is used to denote statistical significance: **p<0.01, *p<0.05.
DPT was purified from the root of Anthriscus sylvestris (Fig. 1A). To assess its cytotoxicity in DCs, cells were treated with various DPT concentrations for 24 h. No marked differences in the percentage of dead cells, as defined by CD11c and Annexin V/propidium iodide staining (Fig. 1B), was observed. However, we found weak dead cells percentage of dendritic cells over 200 nM of DPT treatment (less than 6% of dead cells). Therefore, we decided to use the concentration at less than 100 nM in the following experiments.
Initially, we sought to determine the effects of DPT on the maturation of sentinel DCs into effector DCs. Bone marrow-derived DCs were cultured for six days in medium supplemented with 20 ng/ml each of granulocyte macrophage colony-stimulating factor and IL-4. Different concentrations of DPT were added on day 6 and lipopolysaccharide (LPS) was used as a positive control. We investigated the effects of a range of DPT concentrations on DC maturation. Bone marrow-derived DCs were cultured for 24 h in the presence of 0 to 100 nM DPT as described in Materials and Methods. The DC populations were subsequently analyzed by flow cytometry for expression of cell surface molecules involved in CTL activation, namely the B7 family markers CD80 and CD86, as well as MHC class I and II. Expression of these molecules on DCs increased in response to DPT treatment in a dose-dependent manner (Fig. 2A) and was similar to the response to LPS treatment. In contrast, untreated DCs retained an immature phenotype. Immature DCs are efficient at capturing antigen and undergoing endocytosis. To investigate whether DPT modulates the ability of DCs to perform andtigen endocytosis, dextran uptake was analyzed. Uptake was significantly lower in DPT- and LPS-treated DCs than in untreated immature DCs (Fig. 2B), indicating a decrease in antigen endocytosis by the treated cells.
Previous reports have suggested that DCs, as well as macrophages and monocytes, function as sources of pro-inflammatory molecules (29). In addition, CD4+ T lymphocytes differentiate into varying subsets of effector cells, including Th1 and Th2 cells. The potent factors driving Th1 and Th2 differentiation are IL-12, which is secreted by DCs, and IL-4, which is secreted by T cells (30). We assessed the ability of DCs to produce pro-inflammatory cytokines such as IL-12 and IL-10. IL-12 secretion is a marker for DCs activity and maturation and can be used to promote Th1 cells and induce IFN-γ production. IL-12 is also a maturation factor for CTL (31). The secretion of bioactive IL-12 p70 requires the coordinated expression of two subunits, p35 and p40, which are encoded by two independently regulation (32). We analyzed the production of both intracellular IL-12 p40/p70 and bioactive IL-12 p70 in DCs treated with increasing concentrations of DPT. As shown in Fig. 3A, DCs stimulated with 100nM DPT induced large amounts of IL-12 p40/p70 (29.6±1.6%), whereas IL-10 was only marginally detected above control levels (3.8±0.1%). When supernatants were analyzed by ELISA, IL-10 was not detected above baseline levels 24 h after stimulation with 100 nM DPT. In contrast, ELISA analysis revealed high levels of IL-12 p70 upon stimulation of DCs with DPT (248.1±12.07 pg/ml with DPT versus 96.8±12.8 pg/ml with medium alone) for 24 h (Fig. 3B). These results indicate that DPT exposure induced the DCs to generate large amounts of IL-12 p70 and, by implication, other pro-inflammatory cytokines. These findings also suggest that DPT can induce functional maturation of DCs, which can lead to a DC-mediated Th1 immune response and/or CTL activity.
MAPK activation is important for DC maturation (33). LPS stimulation affects the activation of these signaling pathways in DCs. Fig. 3C shows LPS activation via phosphorylation of p38 and ERK1/2. In order to characterize the effects of DPT on the phosphorylation of these MAPK in DCs, we treated immature DCs with 100 nM DPT. We observed a marked increase in phosphorylation of each kinase. Total ERK1/2 protein was expressed constitutively (Fig. 3C). Studies have also reported other signaling pathways involved in DC maturation, including the NF-κB pathway (21,34). To characterize the role of NF-κB translocation more precisely, we stimulated immature DCs with DPT, then prepared nuclear extracts and evaluated the presence of the NF-κB p65 subunit by western blot. As before, LPS treatment was used as a positive control, and found to enhance nuclear translocation of p65 within 60 min of exposure. Similarly, pretreatment of DCs with 100 nM DPT resulted in p65 nuclear translocation (Fig. 3C). One group of membrane receptors shown to play a key role in the innate immune system and DC activation are the Toll-like receptors (TLR) (35). TLRs are responsible for activating many signaling molecules, including MAPK, NF-κB, and IFN regulatory factors (36,37). For example, TLR signaling in DCs causes upregulation of CD80, CD86, and CD40, as well as induction of IL-12 and IFN-γ, all of which act to drive Th1 and CD8+ T cell activation. Therefore, we proceeded to determine whether DPT led to TLR activation during DC maturation. As seen in Fig. 3D and E, we performed quantitative real-time PCR and antibody staining of TLR4 after stimulation by LPS and DPT. Pretreatment of cells with DPT increased TLR4 expression 1.64-fold, whereas other TLR receptors remained at near undetectable levels.
Increased expression of B7 family members along with MHC I and II in DCs promotes their interaction with and activation of T cells. We investigated whether DPT-treated DCs stimulate proliferation of CD8+ T cells in a syngeneic mixed lymphocyte reaction. DPT-treated DCs derived from wild-type mice stimulated proliferation of T cells derived from OT-1 Tg mice. As shown in Fig. 4A, DCs treated with DPT or LPS exhibited significantly greater proliferation rates than those of the control cells. In fact, the stimulatory effect of DPT was similar to activation by LPS. To determine whether DPT exerts any effect on CD8+ T cell activation, we treated DCs with DPT for 24 h. ELISA analysis of culture supernatants revealed that the amount of IL-2 produced by CD8+ T cells increased following DPT treatment (Fig. 4D). Because DCs are also capable of inducing the polarization of naïve T cells, we evaluated the ability of DPT-activated DCs to induce a Th1 phenotype from naïve CD4+ T cells. CD4+ splenic T cells from DO11.10 Tg mice were co-cultured with DPT-treated DCs derived from wild-type mice in the presence of 1 µM OVA323-339 peptide. ELISA analysis showed that IFN-γ, but not IL-4, secretion increased in response to DPT (Fig. 4B and C). These results suggest that DPT directs CD4+ T cell differentiation towards a Th1 phenotype by inducing IL-12 production.
Following effector stimulation, DCs undergo functional maturation, enter the afferent lymph system, and interact with naïve T cells in order to prime the T cell response (38). Maturation and activation result in changes in the migratory behavior of DCs as a result of altered expression of chemotaxis-related molecules such as CCR7, which is upregulated, and CCR3, which is downregulated (39). We investigated the expression of these chemokine receptors in DPT-treated DCs. LPS was used as a positive control as previous studies have shown that LPS can induce DC maturation via CCR7 upregulation. DCs were treated with DPT for 12 h, after which quantitative real-time PCR was performed to assess CCR3 and CCR7 expression. CCR7 mRNA levels were upregulated whereas CCR3 mRNA was downregulated in DPT-treated DCs (Fig. 5A and B). We next investigated the ability of B16 tumor lysate-pulsed (B16-TP)-DCs and DPT-treated DCs to migrate to draining lymph nodes (DLN) after subcutaneous injection. Both were labeled with CFSE immediately after overnight culture in the presence of B16-TP or DPT, followed by subcutaneous injection into the hind footpads of syngeneic C57BL/6 mice. After 24 h, popliteal DLN were removed and cells were analyzed by flow cytometry to determine the percentage of CFSE positive cells (Fig. 5C). Interestingly, significantly more DPT-treated DCs migrated to the DLNs than untreated DCs and B16-TP-DCs. These results indicate that DPT-treated DCs induce migratory effects in vitro and in vivo.
We first examined whether DPT-treated DCs could enhance immune priming in mice implanted with an established tumor, B16F10 melanoma. As shown in Fig. 6A, mice that received B16-TP-DCs alone showed 40% protection from tumor challenge and survived over 80 days. Immunization with DPT-treated DCs resulted in additional protection from tumor challenge; 80% of these mice survived over time. To determine the therapeutic potential of immunization with DPT-DCs, tumor rejection was assessed in mice implanted with an established B16F10 melanoma. After 28 days, mice that received B16-TP-DCs showed a partial reduction in tumor size (mean=1,325 mm3±372; p<0.001) compared to mice receiving HBSS (mean=2,575 mm3±733) (Fig. 6B, 28 days). Interestingly, tumors in mice that received DPT-treated DCs were significantly smaller than those that received B16-TP-DCs. These findings indicate that DPT can enhance immunization efficacy to protect mice from lethal challenge by the tumor.
DPT-treated DCs induce both increased survival rate and reduction of tumor size in mice in comparison to B16-TP-DCs challenge alone (Fig. 6). DPT-treated DCs therefore increase the antitumor response through enhancement of CTL activity and Th1 immunity. Thus, to determine their effect on host T cell responses, IFN-γ production and CTL activity were analyzed. Lymphocytes isolated from tumor-implanted mice from different groups were co-cultured with mitomycin-inactivated B16F10 cells for 7 days in the presence of IL-2, after which CTL cells were collected. B16F10 melanoma cells were used as target cells. CTL activity was determined using a lactate dehydrogenase release assay at effector: target ratios of 10:1, 25:1, and 50:1. Splenic CTL activity of cells harvested from DPT-treated DCs-injected mice (50:1 ratio=34.1%±2.07 (p<0.001)) was greater than in cells from mice injected with only B16-TP-DCs (50:1 ratio=23.5%±1.26 (p<0.001) (Fig. 6C). To determine whether DPT-treated DCs enhance Th1 cytokine production following intratumoral treatment, IFN-γ and IL-2 secretion from splenocytes derived from tumor-implanted mice were analyzed by ELISA 7 days post-injection. Production of IFN-γ and IL-2 from lymphocytes derived from mice injected with DPT-treated DCs was significantly higher than that from B16-TP-DCs (Fig. 6D and E). Moreover, DPT-treated DCs exhibited greater antitumor activity in comparison to B16-TP-treated DCs and its administration as increased expression of MHC class I and II, as well as CD40, a molecule that interacts with T cells to promote DC maturation, were observed (Fig. 6F). These data indicate that the enhanced antitumor effect of DPT-treated DCs may be involved in increasing the production of Th1 cytokines and CTL activity.
This study demonstrates that DPT elicits a Th1 response by promoting Th1-related cytokine production, including IL-12 and IFN-γ, during DC maturation and activation. Moreover, DPT-treated DCs enhance priming of specific CTL activity, cytokine production, and the therapeutic efficacy of DC-based immunization against an established tumor in vivo. A variety of immunological strategies have been used to elicit strong antitumor effects in an attempt to reduce tumors (8,40,41). Among the various DC-based tumor therapies using specific genes with an antigen transfer system, DC-based immunotherapy focuses on the use of antigens for cytokines, chemokines, co-stimulatory molecules, and MHC class I (42,43). Recently, many studies reported the efficacy of antitumor immunotherapy using antigen- or gene-specific pulsed DCs (44). In addition, we and others have reported that bacterial proteins can induce antitumor activity by increasing DC maturation and CTL activity (8,45).
In this study, we sought to determine whether DPT can elicit DC maturation as an effective adjuvant for DC-based tumor therapy and to evaluate the mechanism involved. We demonstrated that DPT treatment of DCs induced the expression of co-stimulatory molecules and IL-12 production in these cells, thereby inducing CD8+ T cell proliferation, IL-2 and IFN-γ production, as well as Th1-mediated immune responses. DCs maturation and IL-12 production was mediated by signaling through TLR4-mediated MAPK and NF-κB activation. Previous reports suggested that LPS-mediated induction of IL-12 in DCs is mediated by ERK1/2 and p38 (46), which has been linked to enhancement of IL-12 by DCs and ultimately a Th1 response (47). Our results indicate that DPT treatment induces p38 and ERK phosphorylation in DCs, and that induction of IL-12 stimulated IL-2 and IFN-γ production, and promoted a Th1-type response. These results are consistent with previous reports showing that enhancement of a Th1 immune response results in the suppressive effect of tumor-conditioned medium on IL-12 production by DCs (48). Recent studies have also demonstrated that in addition to IFN regulatory factor 5 and NF-κB, other pathways are critical for IL-12p70 production (49).
Generation of a specific CTL response by the immune system may provide a therapeutic approach to cancer. Recent papers have demonstrated that the cytotoxic T cell response can be bypassed by activation of DCs through expression of CD40 (50). DCs also function as a temporal bridge in that, once activated, they are conditioned to deliver a kill signal to CD8+ CTL. Several reports have implicated DCs in this model, suggesting that the activation state of DCs is more important than the help of CD4+ T cells (50). We developed an in vivo model of CTL activity against B16F10 melanoma to test the specific CTL priming capacity of differentially matured DCs. B16-TP-DPT-costimulated DCs induced CTL activity, whereas DPT-matured DCs significantly enhanced antitumor activity when compared to B16-TP-matured DCs. This activity is likely mediated through a T cell interaction molecule such as CD40 and MHC molecules on DCs. Generally, DCs are the exclusive APC capable of mediating capture of Ag from apoptotic cells of tumor for the generation of MHC I/peptide complexes, interaction with CD40 on DCs and CD40L on T cells, thus allowing for engagement of CD8+ T cells activation (50). T cell interaction molecules, including CD40, and MHC class I and II, are expressed at higher levels on DPT-stimulated DCs, allowing increased stimulation of CTL precursors in vivo. DPT-stimulated DCs can then produce more IL-12 and elicit IFN-γ production from T cells, thus expanding CTL.
Our results show that DPT-stimulated DCs injected into C57BL/6 tumor-implanted mice in situ can significantly inhibit tumor growth and prolong survival. Pathologic examination showed that significant tumor necrosis was present inside or around the tumor mass following administration of DPT-treated DCs. These data indicate that injection of DPT-treated DCs into tumor-implanted mice can induce potent antitumor effects.
In summary, our results demonstrate that effective DCs maturation and activation by DPT can produce several beneficial effects when administered as tumor therapy. These include polarization of tumor-reactive T cell responses toward a Th1 profile, increased survival rate and activation of CTL, and IFN-γ production, all of which work with the adaptive immune response in the polarization process. These findings also provide new insight into the immunopharmacology of DPT.
Figures and Tables
Figure 1
DPT is not cytotoxic to DCs. Chemical structures of deoxypodophyllotoxin (DPT) (A) On day 6, the cells were cultured under standard conditions for another 24 h in the presence of 1 nM to 1,000 nM DPT and harvested. The cells were gated on CD11c+ cells, and analyzed by 2-color flow cytometry using AnnexinV/PI staining kit [H2O2 (200 µM positive control)] (B) This result is representative of 3 experiments that gave similar results.

Figure 2
DPT induces the expression of MHC class I and II, co-stimulatory molecules in a dose-dependent manners and decreased endocytic capacity in DCs. The DCs were cultured under standard conditions for 24 h in the presence of 1 to 100 nM DPT or 200 ng/ml LPS, harvested, and analyzed by 2-color cytometry. The cells were first gated on CD11c+. Medium represents the untreated control and LPS represents the positive control. DPT was added to the DCs for 24 h at concentrations of 1 to 100 nM (A) FITC-dextran uptake was analyzed by CD11c+-PE/dextran-FITC-positive cells using flow cytometry. DCs (1×105 cells) were treated with DPT (100 nM) or LPS (200 ng/ml) for 24 h (B) Endocytic activity of the control was determined after incubation at 4℃. The numbers represent the percentage of FITC-dextran/CD11c+-PE double positive cells. Histogram of Fig. 2B was shown (thick line; endocytic activity control at 4℃, thin line: endocytic activity at 37℃).

Figure 3
DPT induces the production of IL-12 through MAPK and NF-κB in DCs. DCs were generated by stimulating immature DCs with 1 to 100 nM DPT or 200 ng/ml LPS (Positive control) for 24 h. After 24 h, the production of IL-12 and IL-10 was measured by flow cytometry (A) Analysis of IL-12 p70 and IL-10 production by magnetic bead-purified DC (1×106 cells) using ELISA. **p<0.01 for a comparison with medium control (B) For MAPK and NF-κB activation analysis, DCs were pretreated with 100 nM DPT for 15, 30, and 45 min. The cell lysates were prepared and blotted with anti-phosho ERK1/2 and anti-phosho-p38 Ab. The nuclear extracts were blotted with anti-p65 Ab. The results are derived from 1 experiment with triplicate samples (C) DCs were pretreated with medium alone, LPS (positive control, 200 ng/ml), and DPT (100 nM) for 6 h. After 6 h, RNA was isolated and evaluated for TLR gene expression by quantitative real-time PCR. **p<0.01 for a comparison with medium control (D). The expression level of TLR4 after 24 h by stimulation was measured by flow cytometry (E).
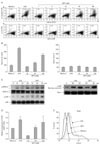
Figure 4
DPT-treated DCs induced the differentiation of T cells to a Th1 response. CD8+ T cell proliferation was analyzed by flow cytometry and presented as the percentage of dividing cells. A negative control (CD8+ T cells in untreated DC alone), a specific Ag control (1 µM OVA257-264) and a positive control (CD8+ T cells in 200 ng/ml LPS) were created for each experiment (A) To analyze cytokine production, CD4+ T cells of DO11.10 Tg mice and CD8+ T cells of OT-1 Tg mice were co-cultured in medium, LPS, or DPT-treated DC with or without OVA at 1:10 DC/T cell ratio for 48 h. After 48 h of expansion, IFN-γ (B), IL-4 (C) and IL-2 (D) were measured by ELISA in culture supernatants. Data are the mean±S.D. of 4 independent experiments. **p<0.01 for a comparison with medium control.

Figure 5
DPT treatment of DCs increased expression of CCR7 and migration to DLN. DCs were treated with medium or DPT (100 nM) for 12 h. After 12 h, RNA was isolated and evaluated for CCR 7 (A) and CCR 3 (B) gene expression by quantitative real-time PCR. Fold differences compared with medium alone are depicted after normalization with the housekeeping gene. **p<0.01 for a comparison with medium control. Fluorescence-activated cell sorter analysis was performed on cells from DLN isolated 24 h after subcutaneous injection of DCs alone, B16-TP-DCs, and DPT treated DCs with 1×106 cells, bead-purified, CFSE-labeled CD11c+ DC. The incidence of labeled DC migration to DLN was expressed as a percent of fluorescent cells (C) This result is representative of 3 experiments that gave similar results. **p<0.01 for a comparison with DC alone.

Figure 6
DPT-DCs enhance anti-tumor therapeutic efficacy. C57BL/6 mice were injected subcutaneously with 1×105 B16F10 cells on day 0. The tumor-implanted C57BL/6 mice were divided into 4 groups (each group containing 10 mice) and injected in situ with DCs alone (◆), B16-TP-DCs (□), DPT-DCs (○), and HBSS (█), respectively. Injection of 1×106 purified DCs/0.2 ml was performed on day 3, 7, and 14. The length and width of the tumor mass were measured with calipers at 3, 7, 14, and 28 days. Tumor-implanted mice in each group were observed for survival time (A) Inhibition of tumor growth in melanoma-implanted C57BL/6 mice by therapy group (B) Splenic lymphocyte isolation from killed tumor-implanted mice (n=5) 5 days after final injection with HBSS, purified DCs, B16-TP-DCs, and DPT-DCs co-cultured with inactivated B16F10 cells for 7 days in the presence of recombinant murine IL-12 (20 U/ml) and then collected as CTL effector cells and incubated at the indicated E:T ratio. CTL activity was determined by LDH release assay with a CytoTox 96 non-radioactive cytotoxicity assay kit (C). ELISA analysis of IFN-g (D) and IL-2 (E) in the supernatants of 2×106/ml lymphocytes isolated at 5 days after the last injection and restimulated with inactivated B16F10 cells in vitro at a 10:1 ratio. The DCs were cultured under standard conditions for another 24 h in the presence of B16-TP or 100 nM DPT, harvested, and analyzed by 2-color cytometry (F). Data are representative of 3 independent experiments. Mice were monitored for tumor growth and survival. (*p<0.05, **p<0.01, DPT-DCs vs. HBSS).
References
1. Tobiásová Z, Pospísilová D, Miller AM, Minárik I, Sochorová K, Spísek R, Rob L, Bartůnková J. In vitro assessment of dendritic cells pulsed with apoptotic tumor cells as a vaccine for ovarian cancer patients. Clin Immunol. 2007. 122:18–27.

2. Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997. 186:65–70.

3. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–296.

4. Morisaki T, Matsumoto K, Onishi H, Kuroki H, Baba E, Tasaki A, Kubo M, Nakamura M, Inaba S, Yamaguchi K, Tanaka M, Katano M. Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell. 2003. 16:175–182.

5. Rock KL, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996. 156:3721–3726.
6. Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000. 30:705–708.

7. Shimizu K, Thomas EK, Giedlin M, Mulé JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001. 61:2618–2624.
8. Jeannin P, Renno T, Goetsch L, Miconnet I, Aubry JP, Delneste Y, Herbault N, Baussant T, Magistrelli G, Soulas C, Romero P, Cerottini JC, Bonnefoy JY. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat Immunol. 2000. 1:502–509.

9. Sloan JM, Kershaw MH, Touloukian CE, Lapointe R, Robbins PF, Restifo NP, Hwu P. MHC class I and class II presentation of tumor antigen in retrovirally and adenovirally transduced dendritic cells. Cancer Gene Ther. 2002. 9:946–950.

10. Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998. 392:86–89.

11. You Z, Hester J, Rollins L, Spagnoli GC, van der Bruggen P, Chen SY. A retrogen strategy for presentation of an intracellular tumor antigen as an exogenous antigen by dendritic cells induces potent antitumor T helper and CTL responses. Cancer Res. 2001. 61:197–205.
12. Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996. 183:283–287.

13. Huang J, Tatsumi T, Pizzoferrato E, Vujanovic N, Storkus WJ. Nitric oxide sensitizes tumor cells to dendritic cell-mediated apoptosis, uptake, and cross-presentation. Cancer Res. 2005. 65:8461–8470.

14. Liu Y, Bi X, Xu S, Xiang J. Tumor-infiltrating dendritic cell subsets of progressive or regressive tumors induce suppressive or protective immune responses. Cancer Res. 2005. 65:4955–4962.

15. Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, Dunne P, Mills KH. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008. 180:3797–3806.

16. Zanoni I, Foti M, Ricciardi-Castagnoli P, Granucci F. TLR-dependent activation stimuli associated with Th1 responses confer NK cell stimulatory capacity to mouse dendritic cells. J Immunol. 2005. 175:286–292.

17. Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004. 173:7454–7461.

18. Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006. 5:471–484.

19. Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006. 12:207–213.

20. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003. 3:984–993.

21. Lee JS, Lee JC, Lee CM, Jung ID, Jeong YI, Seong EY, Chung HY, Park YM. Outer membrane protein A of Acinetobacter baumannii induces differentiation of CD4+ T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem Pharmacol. 2007. 74:86–97.

22. Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004. 101:10116–10121.

23. Kim Y, Kim SB, You YJ, Ahn BZ. Deoxypodophyllotoxin: the cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 2002. 68:271–274.

24. Sudo K, Konno K, Shigeta S, Yokota T. Inhibitory effects of podophyllotoxin derivatives on herpes simplex virus replication. Antivir Chem Chemother. 1998. 9:263–267.

25. Jin M, Moon TC, Quan Z, Lee E, Kim YK, Yang JH, Suh SJ, Jeong TC, Lee SH, Kim CH, Chang HW. The naturally occurring flavolignan, deoxypodophyllotoxin, inhibits lipopolysaccharide-induced iNOS expression through the NF-kappaB activation in RAW264.7 macrophage cells. Biol Pharm Bull. 2008. 31:1312–1315.

26. Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000. 243:147–154.

27. Préville X, Ladant D, Timmerman B, Leclerc C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 2005. 65:641–649.
28. Abe M, Zahorchak AF, Colvin BL, Thomson AW. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation. 2004. 78:762–765.

29. Mosca PJ, Hobeika AC, Clay TM, Nair SK, Thomas EK, Morse MA, Lyerly HK. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000. 96:3499–3504.

30. Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000. 1:311–316.

31. Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995. 13:251–276.

32. Lutz MB, Assmann CU, Girolomoni G, Ricciardi-Castagnoli P. Different cytokines regulate antigen uptake and presentation of a precursor dendritic cell line. Eur J Immunol. 1996. 26:586–594.

33. An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, Liu S, Wang W, Guo Z, Guo J, Qin Z, Cao X. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002. 106:38–45.

34. MacDonald KP, Kuns RD, Rowe V, Morris ES, Banovic T, Bofinger H, O'Sullivan B, Markey KA, Don AL, Thomas R, Hill GR. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood. 2007. 109:5049–5057.

35. Sioud M, Fløisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007. 37:2834–2846.

36. Sweet CR, Conlon J, Golenbock DT, Goguen J, Silverman N. YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell Microbiol. 2007. 9:2700–2715.

37. Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008. 205:315–322.

38. Ato M, Stäger S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002. 3:1185–1191.

39. Catusse J, Spinks J, Mattick C, Dyer A, Laing K, Fitzsimons C, Smit MJ, Gompels UA. Immunomodulation by herpesvirus U51A chemokine receptor via CCL5 and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human leukocytes. Eur J Immunol. 2008. 38:763–777.

40. Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, Rimoldi D, Liénard D, Gugerli O, Ferradini L, Robert C, Avril MF, Zitvogel L, Angevin E. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004. 64:2192–2198.

41. Sancho D, Mourão-Sá D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008. 118:2098–2110.

43. Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R, Melcher AA. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother. 2008. 31:620–632.

44. Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, Mori K, Okada Y, Fujita T, Hayakawa T, Mayumi T, Yamamoto A. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 2001. 61:7913–7919.
45. Lee JS, Kim JW, Choi CH, Lee WK, Chung HY, Lee JC. Anti-tumor activity of Acinetobacter baumannii outer membrane protein A on dendritic cell-based immunotherapy against murine melanoma. J Microbiol. 2008. 46:221–227.

46. Usluoglu N, Pavlovic J, Moelling K, Radziwill G. RIP2 mediates LPS-induced p38 and IkappaBalpha signaling including IL-12 p40 expression in human monocyte-derived dendritic cells. Eur J Immunol. 2007. 37:2317–2325.

47. Kelsall BL, Stüber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996. 795:116–126.
48. Iwashita Y, Goto S, Tominaga M, Sasaki A, Ohmori N, Goto T, Sato S, Ohta M, Kitano S. Dendritic cell immunotherapy with poly (D,L-2,4-diaminobutyric acid)-mediated intratumoral delivery of the interleukin-12 gene suppresses tumor growth significantly. Cancer Sci. 2005. 96:303–307.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download