INTRODUCTION
Interleukin (IL)-21 is a member of the type I cytokine family with significant sequence homology to IL-2, IL-4, and, in particular, IL-15 (1 -3). IL-21 is also the most recently described member of the common γ-chain cytokine family, and found to be a potent immunomodulatory cytokine (4). IL-21 receptor (IL-21R) has been reported to associate with the common γ chain, a property it shares with the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15. The intracellular signaling pathway of IL-21R involves Janus kinase 1 (Jak1), Jak3, signal transducer and activator of transcription 1 (Stat1), Stat3, Stat5a and stat 5b (5-7). IL-21 was first noted to be produced by activated CD4+ T cells, whereas expression of its receptor was somewhat broader (8,9). IL-21 serves a critical role for immunoglobulin production and terminal B cell differentiation, acts as a T cell comitogen and can drive the expansion of CD8+ T cells, can negatively regulate dendritic cell function and plays an essential role in the differentiation of Th17 cells (10). Also, IL-21 is implicated in the pathogenesis of autoimmunity (11,12) and exhibits potent actions as an antitumor agent (13).
The process of new blood vessel formation or angiogenesis is essential for tumor growth and wound healing as it is critical for supply of oxygen and nutrients. Especially, vascular endothelial growth factor (VEGF) has an essential role in angiogenesis. VEGF is thought to play an important role in the increased vascular permeability and angiogenesis associated with tumor malignancies. It augments neovascularization and tumor growth in cells transfected with the VEGF gene versus control cells (14). Overexpression of VEGF and both of its receptors has been documented in a number of animal and human tumors (15). VEGF is also overexpressed by epidermal keratinocytes in certain non-neoplastic processes of the skin which are characterized by increased microvascular permeability and angiogenesis, e.g. cutaneous wound healing (16) and psoriasis (17). Recently, up-regulation of VEGF was also found in bullous pemphigoid, erythema multiforme, and dermatitis herpetiformis (18), which are all associated with hyperpermeable dermal microvessels. In addition, members of the type I cytokine family such as IL-2 and IL-4, which is analogy to IL-21, are suggested to affect the formation of new blood vessels (19-21).
Ultraviolet B (UVB) induces multiple inflammatory and carcinogenic reactions like angiogenesis. In skin or human keratinocyte cells, UVB induces to secrete inflammatory cytokines such as IL-1α, IL-6 and IL-8 (22,23). In addition, cutaneous angiogenesis is enhanced by exposure to UVB irradiation (24) and UVB-induced skin angiogenesis is mediated by upregulating of VEGF (25). In present study, we examined the production and regulation of IL-21 and VEGF by UVB irradiation in a human keratinocyte cell line, HaCaT.
MATERIALS AND METHODS
Cells and UVB irradiation
The human keratinocyte cell line, HaCaT was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI 1640 supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100µg/ml streptomycin, and 10% heat-inactivated fetal bovine serum. This cell line in the log phase of growth was used for experiments. Cells were exposed to UVB irradiation (290~320 nm) using bank of lamps (Waldmann, Schwenningen, Germany). The irradiance of the lamps was measured with a photometer (Waldmann, Schwenningen, Germany).
RT-PCR
Total RNA was isolated from 5×106 cultured cells using easy BLUETM, and cDNA was made using Power cDNA synthesis kit. Then, cDNA was amplified with IL-21 primers (5'-GAG ATC CAG TCC TGG CAA-3', 5'-GCA AGT TAG ATC CTC AGG AA-3'; product=480 bp). Cycling conditions for IL-21 were 1 min at 95℃, 1 min at 56℃, and 1 min at 72℃ for 40 cycles. PCR products were separated on 1.2% agarose gel, stained with ethidium bromide and visualized under UV light.
Flow cytometry
Surface flow cytometry was used to detect the expression of IL-21 receptor (IL-21R). HaCaT cells were collected, washed twice with 0.15 M phosphate-buffered saline (PBS), and incubated with anti-human IL-21R monoclonal antibody (R&D system, Minneapolis, MN, USA). And then cells were incubated with FITC-conjugated anti-mouse IgG (Sigma, St. Louis, MO, USA), and analyzed with flow cytometer. Intracellular flow cytometry was performed to detect the production of IL-21. HaCaT cells were collected after UVB irradiation, washed twice with 0.15 M PBS. After fixing with fixation buffer (2% paraformaldehyde in PBS) for 20 min on ice, cells were permeablized with permeabilization buffer (0.1% saponinin PBS) for 30 min on ice. Then, cells incubated with FITC-conjugated anti-goat IgG (Sigma, St. Louis, MO, USA) for 30 min on ice, washed twice with permeabilization buffer, and analyzed with flow cytometer. Flow cytometric data was obtained using an Epics ALTRA (Beckman coulter, Brea, CA, USA) after processed by the Expo32 program (Beckman coulter, Brea, CA, USA).
RESULTS
Expression of IL-21 and its receptors on the surface of HaCaT cells
It is reported that the interaction between keratinocytes and inflammatory cells are one of the important factors on the pathogenesis of Psoriasis, chronic inflammatory disease of the skin (26). In addition, IL-21 receptor is expressed on a variety of immune cells as well as non-immune cells (27). Therefore, we investigated the expression of IL-21 and its receptor on human keratinocyte cell line, HaCaT by flow cytometry analysis. As shown in Fig. 1, we found the spontaneous IL-21 production and IL-21R expression in HaCaT.
The enhancement of IL-21 production from HaCaT by UVB irradiation
There are several reports regarding the pivotal role of IL-21 in chronic inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and psoriasis. Therefore we investigated whether IL-21 is produced in skin under inflammatory condition by UVB, which can lead inflammatory responses and production of various inflammatory cytokines and chemokines (28). To determine the optimal dose of UVB, the changes of IL-21 mRNA expression were examined after cells were exposure to various ranges of UVB from 100 to 300 J/m2. IL-21 mRNA expression was increased in a dose-dependent manner of UVB. Since IL-21 mRNA expression was peaked under 150 J/m2 of UVB, we investigated IL-21 production at proteins level by intracellular flow cytometry analysis. As shown in Fig. 2B, IL-21 production was increased by UVB irradiation.
The secretion of VEGF was enhance by UVB in HaCaT cells
Angiogenic process is an essential step for the regulation of inflammatory responses. We already reported IL-18 production is up-regulated from HaCaT by UVB irradiation. In addition, IL-18 is a reported as one of the angiogenic factors. Therefore, we investigated whether VEGF expression is regulated by UVB irradiation. As we expected, VEGF production is increased by UVB irradiation in a dose-dependent manner (Fig. 3).
IL-21 production was involved in the VEFG production
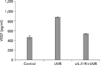
As we have already shown that IL-21 production and its receptor expression and VEGF production is increased by UVB irradiation, we further investigated the relationship between IL-21 and VEGF production by UVB irradiation. When the autocrine effect of IL-21 on UVB-irradiated HaCaT was blocked by soluble IL-21 receptor, we found complete inhibition of UVB-induced VEGF production (Fig. 4).
DISCUSSION
In the present study, we investigated the production of IL-21 and VEGF in a human keratinocyte cell line, HaCaT by UVB irradiation. And IL-21 is involved in the secretion of VEGF by UVB exposure. Interleukin (IL)-21 is a pleotropic cytokine with immune-regulatory properties and immune-therapeutic capacity. A recent study demonstrated that IL-21 receptor is present on normal and tumor endothelial cells and can mediate angiostatic properties (21). Although several inflammatory cytokines such as TNF-α, IL-6 and IL-8 are secreted by UVB (28), there was no report of the production of IL-21 by UVB irradiation. However, we showed the exposure of UVB to human kerationocyte cell line, HaCaT induced significant production of IL-21 and increased the surface expression of IL-21 receptors. Nurieva et al. represented that IL-21 acts as an autocrine cytokine and was involved in the generation of inflammatory T cells (29). Thus, increased expression of both intracellular IL-21 and its surface receptors by exposure to UVB on HaCaT cells (Fig. 1 and 2) is suspected to expand the activity of IL-21.
Vascular endothelial growth factor (VEGF) stimulates the growth of new blood vessels, so it is involved in not only tumor growth and metastasis but also wound healing. VEGF elicits a strong angiogenic response in a variety of in vivo models. VEGF participates in the angiogenic responses by increasing microvascular permeability. Also, VEGF stimulate several endothelial cell responses in cell culture including proliferation, migration, survival, and secretion of matrix-degrading enzymes (14). Acute ultraviolet B (UVB) irradiation of the skin results in erythema, vasodilation, edema, and angiogenesis, which is associated with the expression of VEGF by epidermal keratinocytes (30). Sublethal and physiologically relevant doses of UVB increased VEGF mRNA and protein levels upon irradiation of quiescent keratinocytes. The UVB-induced overexpression of VEGF is dependent on de novo protein synthesis and occurs via release of soluble mediators, which subsequently turn on VEGF expression (31). It is unclear whether VEGF is required for the damage or repair process that occurs in the skin on UVB exposure. However, a study with VEGF-overexpressing transgenic mice reported that VEGF promotes the cutaneous damage that occurs after UVB exposure and the author mentioned VEGF signaling pathway might serve as a novel target for the prevention of UVB-induced photodamage (30). Our result also indicated the elevated secretion of VEGF by UVB irradiation, but the effect of produced VEGF is considered to be further studied.
We also showed that the treatment of soluble IL-21R blocked the production of VEGF by UVB irradiation. It means that VEGF production is modulate by IL-21 and that IL-21 can function in similar to VEGF. The report that co-localization of IL-21R and VEGF was consistently seen in the skin of patients with skin sclerosis (27) is supporting our results. Recently, it is also reported that IL-21 receptor regulates wound healing and fibrosis by controlling Th2-mediated inflammation (32).
In conclusion, a human keratinocyte cell line, HaCaT expressed both IL-21 and IL-21 receptor, and the irradiation of UVB induced the increase of the IL-21 production. Also, the secretion of VEGF is elevated by exposure to UVB, and IL-21 was involved in the VEGF production. It suggests that IL-21 exhibits the angiogenetic activity by modulating VEGF when UVB is exposed to skin keratinocytes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download