Abstract
Background
Although intraoperative opioids provide more comfortable anesthesia and reduce the use of postoperative analgesics, it may cause opioid induced hyperalgesia (OIH). OIH is an increased pain response to opioids and it may be associated with N-methyl-D-aspartate (NMDA) receptor. This study aimed to determine whether intraoperative nefopam or ketamine, known being related on NMDA receptor, affects postoperative pain and OIH after continuous infusion of intraoperative remifentanil.
Methods
Fifty-four patients undergoing laparoscopic cholecystectomy were randomized into three groups. In the nefopam group (N group), patients received nefopam 0.3 mg/kg at the induction of anesthesia followed by a continuous infusion of 0.065 mg/kg/h. In the ketamine group (K group), patients received ketamine 0.3 mg/kg at the induction of anesthesia followed by a continuous infusion of 3 µg/kg/min. The control group did not received any other agents except for the standard anesthetic regimen. Postoperative pain score, first time and number of demanding rescue analgesia, OIH and degrees of drowsiness/sedation scale were examined.
Results
Co-administrated nefopam or ketamine significantly reduced the total amount of intraoperative remifentanil and postoperative supplemental morphine. Nefopam group showed superior property over control and ketamine group in the postoperative VAS score and recovery index (alertness and respiratory drive), respectively. Nefopam group showed lower morphine consumption than ketamine group, but not significant.
Opioids are the agents of choice for the treatment of moderate to severe pain. Opioid is increasingly used during surgery combined with inhalation or intravenous anesthetics since it has many advantages such as blocking adverse reactions to surgical stimulations during surgery, stabilizing the vital signs and reducing the anesthetics dose. In particular, remifentanil has advantages showing rapid onset and short duration of action, thus it is widely used during general anesthesia currently.
However, perioperative opioids can cause several serious problems in a dose dependent manner. The major adverse effect is respiratory depression and hypotension; additionally, delayed recovery from anesthesia, postoperative sedation, nausea and vomiting can be produced by perioperative opioid use. Moreover, in terms of postoperative analgesia, perioperative opioid also can cause acute tolerance and opioid induced hyperalgesia (OIH).
The OIH is a unique phenomenon that increases the pain state from the usage of opioids. It has generally been accepted that OIH is associated with the long term use of opioids such as morphine, hydrocodone, oxycodone, and methadone [12]. However, recent experimental and clinical studies have demonstrated that only a brief opioid exposure can induce the OIH [3456]. Moreover, OIH tends to develop more rapidly and more frequently with the administration of potent short-acting opioids such as remifentanil than with long-acting opioids [7] and a relatively large dose of intraoperative remifentanil has been shown eager to trigger the OIH [8]. Although the mechanisms of this phenomenon are not fully understood, but it has been suggested that activated N-methyl-D-aspartate (NMDA) receptors may play an key role to induce OIH [9]. The administration of opioids can activate pain inhibitory as well as pain facilitatory systems and pain hypersensitivity has been associated with an abnormal predominance of pronociceptive pathways such as sensitization of the NMDA receptor system.
Ketamine is a non-competitive NMDA receptor antagonist [10] that is a widely used in general anesthesia due to its analgesic effects and relatively stable cardiovascular property. Several studies in humans and animals have reported that small-dose ketamine can attenuate central sensitization and may prevent OIH [5811]. Nefopam is a novel non-opioid, non-steroidal, centrally acting analgesic drug with a structure similar to that of an uncompetitive NMDA receptor antagonist [12]. Intravenous nefopam produces potent inhibition of the nociceptive reflex in the human [13]. According to a report by Kapfer et al. [14], both intraoperative ketamine and nefopam enhanced the analgesic effect of morphine and improved postoperative pain control.
We tested the hypothesis that administration of intraoperative ketamine or nefopam reduces total amount of intraoperative remifentanil and therefore prevents and/or attenuates the opioid related problems especially with OIH. We primarily assessed the amounts of opioids used during surgery and postoperative pain control and also determined the development of opioid related side effects and OIH in patients receiving laparoscopic cholecystectomy under general anesthesia using sevoflurane and remifentanil with concomitant infusion of ketamine or nefopam.
This study was performed on approval by the Institutional Review Board of our hospital. The double-blind randomized controlled trial was designed. All patients voluntarily signed the informed consent form. Data were collected from June 2015 to August 2015. Fifty-four patients who underwent laparoscopic cholecystectomy and were classified as categories of 1 or 2 according to the American Society of Anesthesiologists Physical Status (ASA PS) classification system were selected as subjects; all subjects were under 70 years old. The exclusion criteria were as follows: categories of 3 or higher according to the ASA PS classification; under the age of 19; hypersensitivity or resistance to the test drugs; nasogastric tube placement after surgery; participation in other clinical trial using other drugs within 30 days of screening; active liver diseases (HIV infections, hepatitis B or C, positive serologic test), conditions or medical history of organ dysfunction associated with alcohol; excessive alcohol intake or clinically significant alcoholism; expected loss of 500 ml or more of blood during the surgery; and not suitable to participate in the clinical trial. In addition, those who took monoamine oxidase (MOA) inhibitor were excluded, because MAO inhibitor stimulated or inhibited the central nervous system through interactions with narcotic analgesics.
Subjects were randomized and divided into three groups such as nefopam group (N group), ketamine group (K group) and control group (C group). The randomization into one of the three groups was based on computer generated random table. Block randomization with a block size of six and equal allocation was employed to prevent imbalances in treatment assignments. The randomization sequence was generated by a principal investigator. The details of the series were unknown to the investigators, and the group assignments were kept in a set of sealed envelopes that were labeled only with the case number. Prior to surgery, the investigator opened the appropriately numbered envelope to determine the patients treatment and group classification. Ketamine, Nepofam or normal saline were then prepared in equal volume syringes by the nurse and labeled with the case number. All the parties involved including the patients, the surgeon, the anesthesiologists, and the investigator collecting the data were unaware of the study drugs or the patients' group assignments.
Surgery was carried out by the same surgeon. As the pretreatment, midazolam 2 mg and glycopyrrolate 0.2 mg were intramuscularly administered and intravenous (IV) famotidine 20 mg was administered. After a patient was brought to the operating room, heart rates, blood pressure, oxygen saturation and electrocardiogram were measured. Once a bispectral index scale (BIS) monitor was connected, the reference values were measured. Anesthesia was induced in all patients in the same manner. As induction regimens, propofol 1.5 mg/kg was intravenously administered, IV remifentanil 3 ng/ml was administered through the target controlled infusion (TCI) system using the orchestra pump. A muscle relaxant, rocuronium (0.6 mg/kg) was intravenously administered. In the N group, patients received IV nefopam 0.3 mg/kg at the induction of anesthesia followed by a continuous infusion of 0.065 mg/kg/h [15]. In the K group, patients received IV ketamine 0.3 mg/kg at the induction of anesthesia followed by a continuous infusion of 3 µg/kg/min [16]. The control group did not received any other agents except for the standard anesthetic regimen. The tracheal intubation was performed after confirmation that muscles were sufficiently relaxed. Anesthesia was maintained with sevoflurane and remifentanil. Remifentanil was adjusted to maintain around 20% of baseline mean blood pressure. Levels of sevoflurane were adjusted to maintain BIS 40–60. To reduce postoperative pain on the surgical site, 100 mg of 0.5% bupivacaine hydrochloride was injected by surgeon within the fascia and the subcutaneous tissues immediately before abdominal fascia closure. When the skin closure was completed, the infusion of nefopam or ketamine was stopped and remifentanil and sevoflurane was stopped in all groups. Ondansetron 4 mg was given intravenously in all groups at the end of operation to prevent the postoperative nausea and vomiting. To reverse muscle relaxant, glycopyrrolate 0.4 mg and pyridostigmine 10 mg were injected intravenously. Manual ventilation was carried out by maintaining appropriate tidal volume within the range of end tidal CO2 35–45 mmHg through administration of 100% oxygen. Extubation was carried out when the patient responded to verbal instructions, opened eyes and had spontaneous respiration with sufficient tidal volume. Total anesthetic time and time from skin suture to extubation was measured and the doses of remifentanil used during surgery were assessed in all groups.
Once the patient was brought to postanesthesia care unit (PACU), vital signs, the degree of pain, and degrees of drowsiness and sedation were examined at 5, 15, 30, 45 and 60 min. The degrees of pain and drowsiness/sedation were estimated by visual analog scale (VAS) and the Modified Observer's Assessment of Alertness/Sedation (MOAA/S) scale, respectively. When the patient complained of moderate or severe pain (VAS > 4), morphine 2 mg was injected intravenously at 5, 15, 30, 45 and 60 min in PACU. We ruled the presence of OIH if there was persistent or paradoxical escalation of pain after the morphine injection. The first time and number of supplemental analgesia were observed in each groups. In addition, adverse events such as nausea/vomiting, tachycardia, sweating and pruritus were examined. When adverse effects were observed, treatments were as follows: for pruritus, IV phenyramine 4 mg was given; for nausea and vomiting, IV ondansetron 4 mg was given; when profound sedation (3 points or more) occurred, oxygen therapy was performed immediately
The primary endpoint was the pain score (VAS) in the PACU at each time point, and secondary endpoints were opioid consumption, time from last suture to extubation, occurrence of postoperative nausea and vomiting, and state of sedation during the experimental period. Statistical analysis was done using SPSS for Windows software (version 20.0, IBM Corp., Armonk, NY, USA). All data were expressed as mean ± SD. One way analysis of variance (ANOVA) was used to analyze the differences between the three groups. The Fisher exact test and χ2-test was performed for categorical variables. P value < 0.05 was considered statistically significant. The sample size was determined by "G power." The sample size was based on the precedent study, where the mean difference in pain score (VAS) between the ketamine group and the control group was hypothesized as 1.5 and the standard deviation (SD) as 1.5 [17]. To calculate sample size, we assumed VASs at 0 minute in ketamine, nefopam and control group were 1.5, 1.5 and 3.0, and equal standard deviation (1.5) in all groups. With a power of 80% at an α level of 0.05, 16 patients per group were required. Considering the possibility of subject dropouts, 54 patients were planned to be recruited.
Fifty-four patients undergoing laparoscopic cholecystectomy under general anesthesia were assessed for eligibility to enroll in the study. Demographic data did not differ among the groups (Table 1). No patient was dropped during the study. No critical adverse drug reactions were reported in either group.
Table 2 lists total anesthetic time, amount of total and average remifentanil administered during surgery and time required for extubation among the group. Total anesthetic time and amount of remifentanil used during anesthesia was significantly higher in control group, as compared with nefopam and ketamine groups. In the comparison of the average dose of remifentanil (µg/kg/min) among the groups, nefopam group received significantly lower dose of remifentanil during anesthesia than the other groups. Ketamine group showed significantly increased extubation time, as compared with control group.
There were no significant differences in blood pressure and heart rate during surgery among the groups (data not shown). In addition, there were no differences in blood pressure and heart rate among the groups (Fig. 1).
In the postoperative pain score, nefopam group showed significant lower VAS score at 0, 5, 15, 30 minute on PACU compared with the control group (Fig. 2). There are no significant difference of VAS score between the nefopam and ketamine groups, although the nefopam group showed lower average VAS score than the ketamine group.
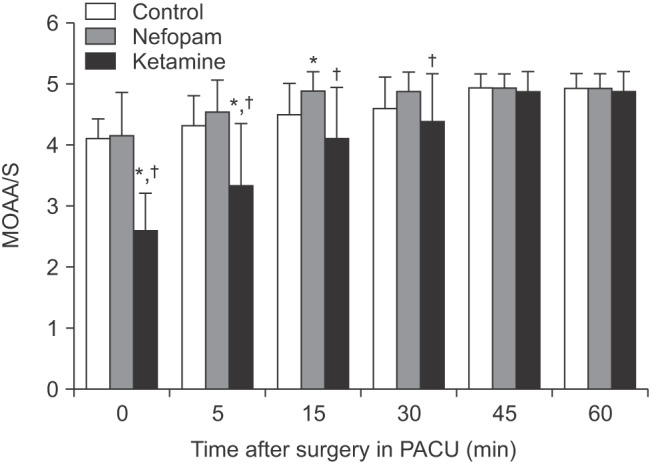
Overall, ketamine group showed lower MOAA/S scale than the other groups at every time point. MOAA/S scale in ketamine group was significantly lower at 0, 5, 15 minute, as compared with control group and 5, 15, 30 minute compared with nefopam group. There were no significant differences in MOAA/S scale between control and nefopam groups (Fig. 3).
OIH was not observed, in all study groups. Every patients who received supplemental morphine showed decreased VAS score, although there were individual differences in efficacy. Table 3 shows postoperative morphine requirement in the PACU. In control group, there were higher request of morphine in regard to the proportion (78% vs. 22% and 44%) and quantitative dosage than the other groups. Additionally, patients in the control group revealed an earlier request time for supplemental analgesia than the other groups. However, there were no significant differences in morphine analgesia between nefopam and ketamine groups. Adverse events in PACU were similar among the groups (Table 4).
OIH was absent in all subjects in this study. The main findings of our study are (1) co-administrated nefopam or ketamine significantly reduced the total amount of intraoperative remifentanil and postoperative supplemental morphine, (2) nefopam group showed superior property over control and ketamine group in the aspect of the postoperative VAS score and recovery (alertness and respiratory drive) after operation, respectively.
OIH is considered a rare phenomenon in the field of clinical anesthesia. However, opioid-based anesthesia is more commonly performed in almost surgeries nowadays and several studies demonstrated that even a brief opioid exposure may associated with the occurrence of OIH [3456]. Additionally, recent systemic review by Kim et al. [18] documented that intraoperative remifentanil ≥ 0.1 µg/kg/min using continuous infusion mode and ≥ 2.7 ng/ml using TCI mode (commonly used in a clinical setting during surgery) seem to be sufficient to develop hyperalgesia.
Opioid-induced hyperalgesia, also called paradoxical hyperalgesia is a phenomenon associated with the chronic use of opioids. Some animal studies have also demonstrated this effect after a single high dose of opioids [19]; in addition, it is associated with too rapid escalation of opioid dosage in palliative care patients [1113]. Therefore, OIH might be induced by the acute exposure to large doses of opioids. Opioids have been considered as first choice to manage moderate or severe pain but opioid alone sometimes provide insufficient analgesia. Based on to the multimodal analgesia, the combination of non-opioid analgesics with opioid provides an opioid sparing-effect and may decrease dose-limiting toxicity such as OIH [20]. Thus, it would be clinically valuable to investigate the pharmacological additives to reduce overall opioid usage and achieve more satisfactory analgesia and thus prevent OIH.
The precise mechanism of OIH remains unknown. However, significant evidence concerning the mechanism and pathophysiology of OIH has been accumulating in the literature. Hyperalgesia may be activated by NMDA receptors, which creates neuronal plasticity and induces central sensitization. Moreover, acute receptor desensitization via uncoupling of the receptor from G proteins, increasing calmodulin-dependent protein kinase II, and upregulation of the cyclic adenosine monphosphate pathway have been suggested as potential mechanisms underlying OIH [21]. Although the mechanism of remifentanil action on NMDA is controversial, activation of NMDA receptor is known to play an important role in remifentanil-induced hyperalgesia [11].
Ketamine is an antagonist of the NMDA receptor and has been known to reduce the remifentanil-induced hyperalgesia in previous studies. According to the study of Hong et al. [22], when low-dose ketamine continued to be injected while sevoflurane and remifentanil were maintained in the laparoscopic gynecologic surgery, early postoperative pain and the quality of opioids used were reduced. In addition, according to the study of Remérand et al. [23], when ketamine was injected at 0.5 mg/kg during the total hip arthroplasty surgery and maintained at 2 µg/kg/min for 24 hours, morphine-sparing effect was shown. It also facilitated rehabilitation at 1 month and decreased postoperative chronic pain up to 6 months after surgery. The mechanism of how ketamine reduces OIH is unknown, but ketamine reduces the usage of opioid and so ketamine is effective in reduction of postoperative pain.
Nefopam has been widely used in many countries, although there is limited clinical data on its use in the treatment of acute and chronic pain. The exact mechanism of action of nefopam remains unclear. However, it is a centrally-acting antinociceptive compound with supraspinal and spinal sites of action and does not bind to opiate receptors [14]. It inhibits monoamine reuptake, modulates descending serotoninergic pain [24], and may interact with a dopaminergic pathway [25] and NMDA receptors [26]. Through these mechanism, nefopam has been known to effective for control acute postoperative pain [27] and also for hyperalgesia [28]. It has shown that nefopam has comparable morphine-sparing effect (30–50%) as ketamine [29].
In this study, there were significant opioid sparing effects in both nefopam and ketamine groups, as compared with the control group. However, significant VAS score decline beside the control group was shown only in the nefopam group. Moreover, nefopam showed superiority over ketamine in the postoperative sedative profile. Nefopam group showed shorter extubation period from end of surgery and higher MOAA/S scale on the PACU. The non-opioid nature of nefopam suggests its fewer potential side effects (such as sedation or respiratory depression). Although not investigated in this study, known psychotomimetic side effects associated with ketamine may limit their clinical potential as adjuvants to the treatment of pain.
The study had some limitations. First, in terms of methodology, there were some validated tests for presence of OIH such as cold compression test, touch sensory threshold test and mechanical allodynia test using Von Frey filament. However, we could not perform these tests due to ethical concerns. We ruled the presence of OIH if there was persistent or paradoxical escalation of pain after supplemental morphine injection. However, it is a non-validated method to judge the OIH and presence of the acute opioid tolerance cannot be ruled out. Second, operation time in control group was significantly longer than the other groups, possibly due to the higher remifentanil consumption during surgery and extubation and recovery time that may have influenced the postoperative pain score. Third, although there were significant opioid sparing effects on nefopam and ketamine group, we could not determine whether the nefopam or ketamine could prevent OIH because no patient showed OIH in the control group in our study design. Fourth, we observed the parameters in PACU only, thus we could not validate the long term effect of experimental drugs. More extensive information on preventing opioid related problems could be obtained if we took the data in the ward. Fifth, the effect of ketamine on BIS. Ketamine is known to raise BIS. In order to maintain BIS 40–60, the concentration of sevoflurane may have been higher and this could affected the postoperative consciousness in ketamine group. However recent studies have shown that low dose ketamine did not affect BIS [30]. Thus, in the present study, ketamine may little affect BIS.
Despite the limitations, this study has worth about evaluating the influences of nefopam and ketamine on postoperative analgesia, opioid consumption and recovery profile after remifentanil based anesthesia. Consequently, nefopam is preferable to ketamine in terms of the VAS score and sedation scale. Future studies are needed to investigate the pharmacological modulation to reverse the OIH.
References
1. Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000; 20:237–245. PMID: 11027904.

2. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001; 63:139–146. PMID: 11376918.

3. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009; 12:679–684. PMID: 19461836.
4. Zissen MH, Zhang G, McKelvy A, Propst JT, Kendig JJ, Sweitzer SM. Tolerance, opioid-induced allodynia and withdrawal associated allodynia in infant and young rats. Neuroscience. 2007; 144:247–262. PMID: 17055659.

5. Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: preventive effect of ketamine. Br J Anaesth. 2010; 104:231–238. PMID: 20031953.

6. Colvin LA, Fallon MT. Opioid-induced hyperalgesia: a clinical challenge. Br J Anaesth. 2010; 104:125–127. PMID: 20086062.

7. De Baerdemaeker LE, Jacobs S, Pattyn P, Mortier EP, Struys MM. Influence of intraoperative opioid on postoperative pain and pulmonary function after laparoscopic gastric banding: remifentanil TCI vs sufentanil TCI in morbid obesity. Br J Anaesth. 2007; 99:404–411. PMID: 17606479.

8. Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005; 103:147–155. PMID: 15983467.

9. Wilder-Smith OH, Arendt-Nielsen L. Postoperative hyperalgesia: its clinical importance and relevance. Anesthesiology. 2006; 104:601–607. PMID: 16508408.
10. Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007; 21:259–271. PMID: 17591654.
11. Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009; 5:76. PMID: 20042082.

12. Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005; 28:183–191. PMID: 15714253.

13. Guirimand F, Dupont X, Bouhassira D, Brasseur L, Chauvin M. Nefopam strongly depresses the nociceptive flexion (R(III)) reflex in humans. Pain. 1999; 80:399–404. PMID: 10204754.

14. Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005; 100:169–174. PMID: 15616073.

15. Richebé P, Picard W, Rivat C, Jelacic S, Branchard O, Leproust S, et al. Effects of nefopam on early postoperative hyperalgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2013; 27:427–435. PMID: 23063945.

16. Lee MH, Chung MH, Han CS, Lee JH, Choi YR, Choi EM, et al. Comparison of effects of intraoperative esmolol and ketamine infusion on acute postoperative pain after remifentanil-based anesthesia in patients undergoing laparoscopic cholecystectomy. Korean J Anesthesiol. 2014; 66:222–229. PMID: 24729845.

17. Hang LH, Shao DH, Gu YP. The ED50 and ED95 of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy. Swiss Med Wkly. 2011; 141:w13195. PMID: 21557114.

18. Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 2014; 5:108. PMID: 24847273.

19. Célèrier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001; 21:4074–4080. PMID: 11356895.

20. Kehlet H, Dahl JB. The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesth Analg. 1993; 77:1048–1056. PMID: 8105724.

21. Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010; 30:38–46. PMID: 20053885.
22. Hong BH, Lee WY, Kim YH, Yoon SH, Lee WH. Effects of intraoperative low dose ketamine on remifentanil-induced hyperalgesia in gynecologic surgery with sevoflurane anesthesia. Korean J Anesthesiol. 2011; 61:238–243. PMID: 22025947.

23. Remérand F, Le Tendre C, Baud A, Couvret C, Pourrat X, Favard L, et al. The early and delayed analgesic effects of ketamine after total hip arthroplasty: a prospective, randomized, controlled, double-blind study. Anesth Analg. 2009; 109:1963–1971. PMID: 19923527.

24. Hunskaar S, Fasmer OB, Broch OJ, Hole K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur J Pharmacol. 1987; 138:77–82. PMID: 2442003.
25. Esposito E, Romandini S, Merlo-Pich E, Mennini T, Samanin R. Evidence of the involvement of dopamine in the analgesic effect of nefopam. Eur J Pharmacol. 1986; 128:157–164. PMID: 3098570.

26. Gray AM, Nevinson MJ, Sewell RD. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. Eur J Pharmacol. 1999; 365:149–157. PMID: 9988097.

27. Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008; 101:610–617. PMID: 18796441.

28. Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001; 44:541–545. PMID: 11735363.

29. McLintock TT, Kenny GN, Howie JC, McArdle CS, Lawrie S, Aitken H. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: a study using patient controlled analgesia. Br J Surg. 1988; 75:779–781. PMID: 3167526.

30. Sengupta S, Ghosh S, Rudra A, Kumar P, Maitra G, Das T. Effect of ketamine on bispectral index during propofol--fentanyl anesthesia: a randomized controlled study. Middle East J Anaesthesiol. 2011; 21:391–395. PMID: 22428494.
Fig. 1
Mean blood pressure (MBP) and heart rates in recovery room. There were no significant differences among the groups at every time point measurement. PACU: postanesthesia care unit.
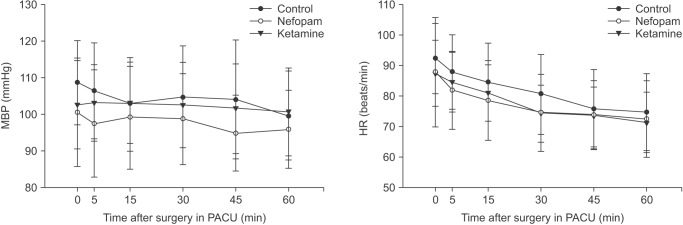
Fig. 2
Pain scores in recovery room. *P < 0.05 compared to control group. Nefopam group showed significant lower visual analog scale (VAS) score at 0, 5, 15, 30 minute on postanesthesia care unit (PACU) compared with control group.
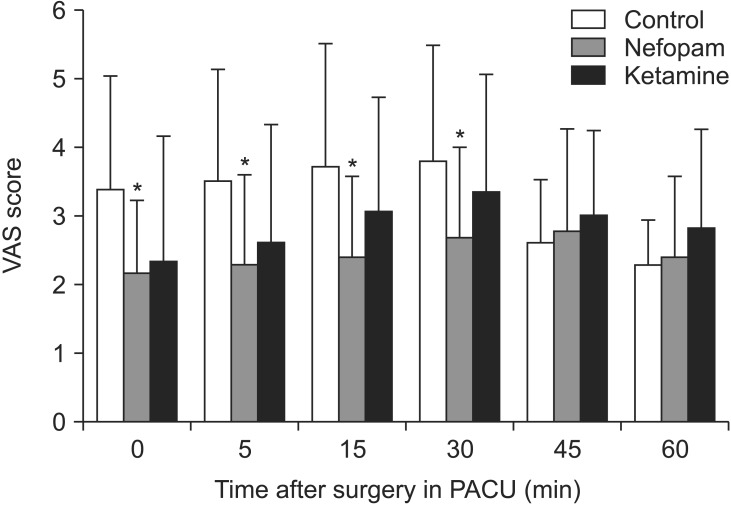
Fig. 3
Modified observer's assessment of alertness/Sedation (MOAA/S) scale in recovery room. MOAA/S scale in ketamine group was significantly lower at 0, 5, 15 minute compared with control group and 5, 15, 30 minute compared with nefopam group. *P < 0.05 compared to control group, †P < 0.05 compared to nefopam group.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download