Abstract
Background
The purpose of this study was to evaluate the effect of intraoperative dexmedetomidine sedation on patient's and surgeon's satisfaction during retinal surgery under sub-tenon's anesthesia.
Methods
Forty-four patients scheduled for elective retinal surgery under sub-tenon's anesthesia were enrolled in this randomized controlled trial. The patients were divided into Dexmedetomidine (n = 22) and Control (n = 22) groups. Intravenous dexmedetomidine or 0.9% saline via infusion pump were administered continuously to the dexmedetomidine or control group, respectively. Ramsay sedation scale with a target level of 3-4 was used to assess adequacy of sedation. Perioperative pain, hemodynamic and respiratory data were collected, while satisfaction from patients and surgeon were assessed post-surgery using a 5-point satisfaction scale.
Results
Patient and surgeon satisfaction was higher in the dexmedetomidine group (P < 0.001, P = 0.002, respectively). The pain associated with sub-tenon's anesthesia and peripheral vitrectomy was lesser in the dexmedetomidine group than in the control group (P = 0.020). There was significant reduction of heart rate in the dexmedetomidine group (P = 0.001), but only one patient needed treatment with atropine. There was no respiratory effect on both groups.
Although local anesthesia or regional anesthesia is preferred in ophthalmic surgery compared to general anesthesia, there are still several adverse effects such as pain, fear and anxiety [12].
Various sedative drugs such as propofol, midazolam, and opioids have been used for retinal surgery. However, these drugs have several adverse effects including respiratory depression, cardiovascular depression, over-sedation, disorientation and may interfere with patient cooperation during surgery [345].
Dexmedetomidine is a strong selective, specific α2-adrenergic agonist that has dose-related sedative and analgesic properties without causing respiratory depression [67]. Its mechanism of action is similar to natural sleep with hyperpolarization of norepinephrine receptor in the locus ceruleus [89]. Unlike other sedatives with significant respiratory depression, dexmedetomidine can enable patients to cooperate during sedation without respiratory depression [10]. In addition, several studies have reported decreased intraocular pressure [11] and delirium preventive property with dexmedetomidine [12]. It is possible for dexmedetomidine to cause bradycardia along with increased blood pressure that subsequently decreases because of its adrenergic effect. However, in patients with history of cardiovascular disease such as hypertension, its hemodynamic effects may be an advantage because of the hemodynamic stability that dexmedetomidine can offer during surgical stress [13].
Studies using intra-operative dexmedetomidine sedation have increased among surgical specialties particularly concerned with respiratory suppression, such as airway surgery and dental surgery [141516]. With cataract surgery, dexmedetomidine sedation has shown increased surgeon and patient's satisfaction [17]. However there are only a few studies looking at intraoperative dexmedetomidine sedation for retinal surgery. Dexmedetomidine sedation in retinal surgery has shown similar hemodynamic effects without respiratory suppressions compared with propofol sedation [18]. However, there was no control-arm in the said retinal surgery study. Therefore this study was conducted with a control group to better assess the improved satisfaction, as well as the hemodynamic and respiratory effects due to dexmedetomidine sedation.
This prospective randomized controlled trial was approved by our hospital's Institutional Review Board. Forty four patients scheduled for elective retinal surgery under sub-tenon's anesthesia were included in this study. Patients aged above 20 and below 65 were included. Participants were excluded if they had histories of severe cardiovascular diseases (ischemic heart disease, congestive heart disease, arrhythmia) and respiratory diseases (asthma, chronic obstructive pulmonary disease etc.), sleep apnea syndrome, dementia or communication difficulties, pregnant women, hypovolemia, bradycardia (heart rate < 50), or hypotension (systolic blood pressure < 90 mmHg).
Informed consent was obtained from all patients and were randomized (computer generated) into two group; Dexmedetomidine group (n = 22) and Control group (n = 22). At arrival in the operating room, non-invasive vital signs were measured including blood pressure, ECG, and pulse oximetry. Oxygen was administered at 2 L/min through nasal cannula with expired CO2 measurement and EtCO2 continuously measured if possible. All measurements were repeated every 10 minutes during the operation. The dexmedetomidine group was loaded with intravenous dexmedetomidine 1 µg/kg over 10 minutes using an infusion pump and subsequently received continuous infusion of dexmedetomidine at 0.4 µg/kg/hr. State of sedation was evaluated every 10 minutes using the Ramsay sedation scale and the dexmedetomidine infusion rate was adjusted accordingly by increasing or decreasing the dose by 0.1 µg/kg/hr. The control group received intravenous saline solution. Both groups were made calm with words to facilitate patient cooperation with the surgical procedures. After injecting the experimental drug, a drop of topical 4% lidocaine was administered to numb the surface of the eye. The patient was then asked to look downwards and to the right. The conjunctiva and Tenon's fascia together were lifted up with forceps and through which a tiny incision was made with the Westcott scissors. A blunt 27-gauge cannula was inserted through this opening and was passed along the globe towards the posterior sub-tenon's space of the eye. Lidocaine (2 ml of 4%) was then injected through this cannula. After local anesthesia, a standard three-port pars plana vitrectomy was performed using either a 23-gauge or 25-gauge vitrectomy system. Cataract surgery was performed along with vitrectomy if needed. All of the pinpoint anesthesia and operations were done by a single surgeon.
Sedation status of the patient was measured every 10 minutes using the Ramsay sedation scale. A Ramsay sedation scale of 3-4 was targeted. In addition, we assessed the pain felt from the anesthetic block and scleral indentation using a 5 point scale (none, mild, moderate, severe, extremely severe). Primary outcome was patient and surgeon satisfaction with the operative experience using a 5 point satisfaction scale (patient's satisfaction scale: 0 = extremely dissatisfied, 1 = dissatisfied, 2 = neither satisfied nor dissatisfied, 3 = satisfied, 4 = extremely satisfied; surgeon's satisfaction scale: 0 = extremely poor, 1 = poor, 2 = fair, 3 = good, 4 = excellent). Assessment of primary outcome was made after the surgery by a trained nurse not involved in this study. Secondary outcomes included hemodynamic changes (blood pressure and heart rate) and respiratory changes (oxygen saturation, EtCO2, respiratory rate) of patients which were recorded by the anesthesiologist during surgery.
Intravenous ephedrine 4 mg was injected when the systolic blood pressure drops below 90 mmHg during sedation. Intravenous atropine 0.5 mg was injected when the heart rate slows to less than 40 beats/min during sedation. The patient was encouraged to breathe and oxygen supply was increased to 4 L/min when oxygen saturation drops to less than 90% or the respiratory rate slows to less than 10 breaths/min.
The estimated sample size was calculated based on the difference in satisfaction scores in a prior study by Erdumus M (3.41 ± 0.80 vs 2.36 ± 1.26) [17]. We assumed an expected difference in satisfaction scores of 1, 20 patients per group was calculated with a two sided α = 0.05 and power of 80% and with an estimated drop-out rate of 10%. Twenty-two persons per group were then planned. Statistical analyses were performed using SPSS version 14.0 for Windows. The two groups were compared using a student's t-test or Mann-Whitney rank-sum test for age, weight, duration of surgery, pre-operative systolic and diastolic arterial pressure, heart rate, respiratory rate and EtCO2, and SPO2. Chisquare analysis or Fisher's exact test for gender, patient's sedation score, patients' and surgeon's satisfaction scale after surgery, pain scale during surgery. Intraoperative blood pressure and heart rate were plotted with graphs and evaluated using repeated measures analysis of variance (ANOVA). Bonferroni test was done if the results of the ANOVA were statistically significant. All data are presented as mean ± standard deviation or Median with interquartile range (IQR) and frequency with percent for categorical data, with statistical significance determined at P < 0.05.
Of the 44 patients enrolled, 2 from the control group dropped out because of bradycardia (heart rate < 50) upon arrival at the operating room. The study ended up with 22 patients included in the dexmedetomidine group and 20 patients included in the control group. The baseline characteristics, preoperative blood pressure, heart rate among the groups and operation times were similar in between groups (Table 1).
Preoperative sedation scale in both groups was similar at 1-2. Intraoperative sedation scale in the dexmedetomidine group was higher than the control group (P < 0.001) (Table 2). Among the patients in the dexmedetomidine group, 13 (59.1%) reached the target intraoperative sedation value of 3-4 while 9 patients (40.9%) were over-sedated with intraoperative sedation value of 5-6. Patient's satisfaction with the operative experience was higher in the dexmedetomidine group than the control group (P < 0.001) (Table 3). Surgeon's satisfaction with the operative experience was higher in the dexmedetomidine group than control group (P = 0.002) (Table 4). The pain scale during the local anesthetics injection and scleral indentation was significantly lower in the dexmedetomidine group (P = 0.020) (Table 5).
The average changes in blood pressure and heart rate are shown in Figs. 1. and 2. Intra-operative systolic and diastolic blood pressure did not fluctuate significantly in both groups. However, there was significant difference in intraoperative systolic and diastolic blood pressure between groups (P < 0.05). Rescue drug for hypotension was not required in all cases. There was significant reduction in the heart rate during the intraoperative period compared with baseline in the dexmedetomidine group (P = 0.001). Intravenous atropine 0.5 mg was injected in one case of the dexmedetomidine group due to the heart rate dropping below 40 beats/min. There was significant difference in the heart rate between the groups (P < 0.001).
Respiratory parameters such as oxygen saturation, respiratory rate, EtCO2 were not depressed in both groups (Table 6).
This study demonstrated that dexmedetomidine sedation is appropriate in retinal surgery under sub-tenon's anesthesia and improved patient and surgeon satisfaction without respiratory complication.
Intraoperative sedation aims to alleviate anxiety and pain from unpleasant stimuli without respiratory suppression [19]. However, frequently used sedatives, such as propofol and midazolam, act as GABA receptor agonists and they have hemodynamic and respiratory depressive properties with potential for over-sedation. They can make patients reach an uncooperative state [345]. This is a huge disadvantage for ophthalmic surgery. A sedative with less of the above mentioned effects may be better since patients undergoing retinal surgery frequently have comorbidities such as cardiovascular disease, diabetes and they often have severe anxiety because the operation is performed with an opened eye [2].
Dexmedetomidine is a strong selective and specific α2-adrenergic agonist. And it has a different mechanism of sedation compared to the earlier and more commonly used sedatives. Dexmedetomidine cause hyperpolarization of norepinephrine receptor in the locus ceruleus and its mechanism of action is similar to natural sleep [89]. There is clinical evidence that electroencephalography pattern with dexmedetomidine sedation is comparable to non-rapid eye movement sleep [20]. Dexmedetomidine sedation seems to offer better quality of sleep, enabling the patients to remain calm, comfortable and very cooperative without any respiratory suppression. Although dexmedetomidine was approved by the Food and Drug Administration (US FDA) in 1999 as a sedative for mechanically ventilated patients in the intensive care unit [21], its application has gradually expanded into intraoperative sedative purpose. In 2008, it was approved as a sedative for surgery or other procedures [21]. Studies involving intra-operative dexmedetomidine sedation has increased in several clinical departments handling cases susceptible to respiratory suppression and where patient cooperation is necessary especially in airway, sleep apnea, facial surgery and dental surgery [141516].
Ophthalmic surgery is an area of facial surgery that commonly encounters respiratory suppression and where in patient cooperation is essential. Recently there are several studies for dexmedetomidine sedation in cataract surgery. Erdurmus et al. [17] reported that dexmedetomidine sedation enhanced patient and surgeon satisfaction compared to the control group in cataract surgery. Our study showed both patient and surgeon satisfaction was enhanced similar to Erdurmus et al.'s study. A study evaluating dexmedetomidine compared to propofol sedation during retinal surgery reported higher patient satisfaction with dexmedetomidine sedation compared to propofol sedation. The improved patient satisfaction may be related to the sedation induced by dexmedetomidine being similar to natural sleep. The surgeons satisfaction was, however, similar in both group [18].
One of the notable points in our study was that the surgeon's satisfaction was related to the patient's muscle tone during the procedure. The patient's level of muscle relaxation was an important determinant of the surgeon's satisfaction with the surgery. The surgeon opined that excessive tension of the patient's muscles made the operation more difficult and exhausting even with adequate patient cooperation during surgery without sedation. The surgeon was still relatively dissatisfied with the operation even in cases where patients were satisfied with the procedure without sedation. We believe that dexmedetomidine sedation created a better operative condition and improved the surgeon's satisfaction in this study.
Dexmedetomidine also has a dose-relative analgesic property [622]. We anesthetized the patients' eyes with sub-tenon's anesthesia, a widely used technique in cataract, vitreoretinal, strabismus, glaucoma surgery and in many other ophthalmic surgeries. It is reported to be as effective as retrobulbar anesthesia in vitreoretinal surgery [23], and also an efficient and safe anesthetic technique in cataract, trabeculectomy, and vitrectomy [24]. Thorough peripheral vitrectomy was performed in every patient by scleral indentation which caused the most pain and discomfort during the surgery. Patients almost always experience some level of discomfort and pain at the time of local anesthetics injection and scleral indentation despite the subtenon's anesthesia in retinal surgery. Our results showed significantly lower pain scale in the dexmedetomidine group which was consistent with the results of previous studies. We believe this effect may provide an additional advantage when using dexmedetomidine for sedation in ophthalmic surgery.
In previous studies, rapid loading dose injection of dexmedetomidine resulted in a temporary increase in blood pressure which subsequently decreased by virtue of its action on the α2-adrenergic receptor [25]. Patients in the dexmedetomidine group in our study also showed a trend towards a slight albeit temporary increase in blood pressure after the loading dose injection compared to their baseline blood pressure. The blood pressure subsequently stabilized during the surgery. This change in blood pressure was not statistically significance. Dexmedetomidine can provide hemodynamic stability in patients with cardiovascular disease, such as hypertension, by preventing an increase in blood pressure due to surgical stimulus [13]. However, in this study, heart rate was significantly decreased in dexmedetomidine group immediately after the loading dose infusion, and one patient in the dexmedetomidine group required atropine. The α2-adrenergic agonist induced bradycardia is a well-known phenomenon and is its most notable side effect [26]. The α2-adrenergic agonists also have sympatholytic and vagal mimetic effect [27]. Hemodynamic monitoring of heart rate and blood pressure is important during dexmedetomidine sedation.
Unlike other sedatives, dexmedetomidine cause sedation by a mechanism similar to natural sleep which is by hyperpolarization of norepinephrine receptors in the locus ceruleus [8]. This mechanism has also been considered in maintaining respiratory function. In previous studies dexmedetomidine did not cause a direct decrease in respiration and ventilation. Therefore there is little effect on the respiratory responses to hypercapnia and hypoxia [62829]. Our result showed no oxygen desaturation and respiratory complications in both groups. Likewise, previous study comparing dexmedetomidine with propofol sedation showed respiratory rate and oxygen saturation to be higher in the dexmedetomidine group [1830].
It has been reported that dexmedetomidine can reduce intraocular pressure [11], which may be advantageous during retinal surgery. Intraocular pressure was not evaluate in this study but another study exploring cataract surgery indicated no difference in intraocular pressure in their study groups [17]. Therefore, more studies on intraocular pressure during retinal surgery and dexmedetomidine sedation may be required.
In conclusion, dexmedetomidine sedation during retinal surgery improves satisfaction from both patients and surgeon without respiratory complication. It is safe and can become a preferred sedative for retinal surgery. Nonetheless when dexmedetomidine is used, hemodynamic monitoring is required including an increased awareness of possibly using atropine in the management of bradycardia.
References
1. Woo JH, Au Eong KG, Kumar CM. Conscious sedation during ophthalmic surgery under local anesthesia. Minerva Anestesiol. 2009; 75:211–219. PMID: 18987568.
2. Au Eong KG, Tan CS, Ang CL, Lee SS, Venkatesh R, Muralikrishnan R, et al. Intraoperative visual experiences of cataract patients can be both pleasant and unpleasant. Br J Ophthalmol. 2005; 89:1386. PMID: 16170152.

3. Salmon JF, Mets B, James MF, Murray AD. Intravenous sedation for ocular surgery under local anaesthesia. Br J Ophthalmol. 1992; 76:598–601. PMID: 1420042.

4. Weinbroum AA, Szold O, Ogorek D, Flaishon R. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol. 2001; 18:789–797. PMID: 11737177.

5. Cote GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010; 8:137–142. PMID: 19607937.
6. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000; 90:699–705. PMID: 10702460.

7. Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008; 4:619–627. PMID: 18484919.

8. Aghajanian GK, VanderMaelen CP. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982; 215:1394–1396. PMID: 6278591.
9. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008; 21:457–461. PMID: 18660652.

10. Alhashemi JA, Kaki AM. Dexmedetomidine in combination with morphine PCA provides superior analgesia for shockwave lithotripsy. Can J Anaesth. 2004; 51:342–347. PMID: 15064262.

11. Abdalla MI, Al Mansouri F, Bener A. Dexmedetomidine during local anesthesia. J Anesth. 2006; 20:54–56. PMID: 16421680.

12. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009; 301:489–499. PMID: 19188334.
13. Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med. 2003; 114:742–752. PMID: 12829201.

14. Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia. 2007; 62:1132–1138. PMID: 17924894.

15. Busick T, Kussman M, Scheidt T, Tobias JD. Preliminary experience with dexmedetomidine for monitored anesthesia care during ENT surgical procedures. Am J Ther. 2008; 15:520–527. PMID: 19127135.

16. Taghinia AH, Shapiro FE, Slavin SA. Dexmedetomidine in aesthetic facial surgery: improving anesthetic safety and efficacy. Plast Reconstr Surg. 2008; 121:269–276. PMID: 18176230.

17. Erdurmus M, Aydin B, Usta B, Yagci R, Gozdemir M, Totan Y. Patient comfort and surgeon satisfaction during cataract surgery using topical anesthesia with or without dexmedetomidine sedation. Eur J Ophthalmol. 2008; 18:361–367. PMID: 18465717.

18. Ghali A, Mahfouz AK, Ihanamaki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-Tenon's anesthesia. Saudi J Anaesth. 2011; 5:36–41. PMID: 21655014.

19. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002; 96:1004–1017. PMID: 11964611.
20. Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, Snapir A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008; 52:289–294. PMID: 18005372.

21. Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009; 43:2064–2074. PMID: 19934395.

22. Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004; 98:153–158. PMID: 14693611.

23. Lai MM, Lai JC, Lee WH, Huang JJ, Patel S, Ying HS, et al. Comparison of retrobulbar and sub-Tenon's capsule injection of local anesthetic in vitreoretinal surgery. Ophthalmology. 2005; 112:574–579. PMID: 15808246.

24. Roman SJ, Chong Sit DA, Boureau CM, Auclin FX, Ullern MM. Sub-Tenon's anaesthesia: an efficient and safe technique. Br J Ophthalmol. 1997; 81:673–676. PMID: 9349156.

25. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992; 77:1134–1142. PMID: 1361311.
26. Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999; 54:1136–1142. PMID: 10594409.

27. Lipscombe D, Kongsamut S, Tsien RW. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989; 340:639–642. PMID: 2570354.
29. Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000; 4:302–308. PMID: 11056756.
30. Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002; 95:461–466. PMID: 12145072.

Fig. 1
Intraoperative systolic and diastolic blood pressure changes between the groups. Values are presented as mean ± standard deviation. There were significant differences between groups in the systolic and diastolic blood pressure (Respectively P = 0.002, P < 0.001). No significant fluctuation was seen in the intra-operative systolic and diastolic blood pressure in both groups. Control: Control group, Dex: Dexmedetomidine group. T0: baseline, T1: Loading dose infusion time, T2: 10 min after loading dose infusion, T3: Starting time of operation, T4: 10 min, T5: 20 min, T6: 30 min after starting operation. *P < 0.05, †P < 0.001 compared with control group.
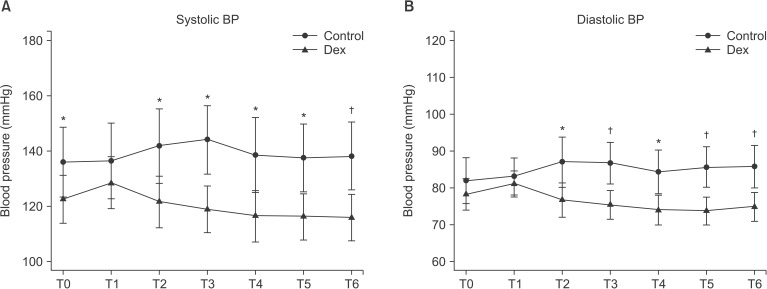
Fig. 2
Perioperative heart rate changes between the groups. There was significant reduction of heart rate in the intraoperative period compared with baseline in the dexmedetomidine group (P < 0.001) but not in the control group. There was significant difference in between groups (P < 0.001). Control: Control group, Dex: Dexmedetomidine group. T0: baseline, T1: Loading dose infusion time, T2: 10 min after loading dose infusion, T3: Starting time of operation, T4: 10 min, T5: 20 min, T6: 30 min after starting operation. *P < 0.001 compared with control group.
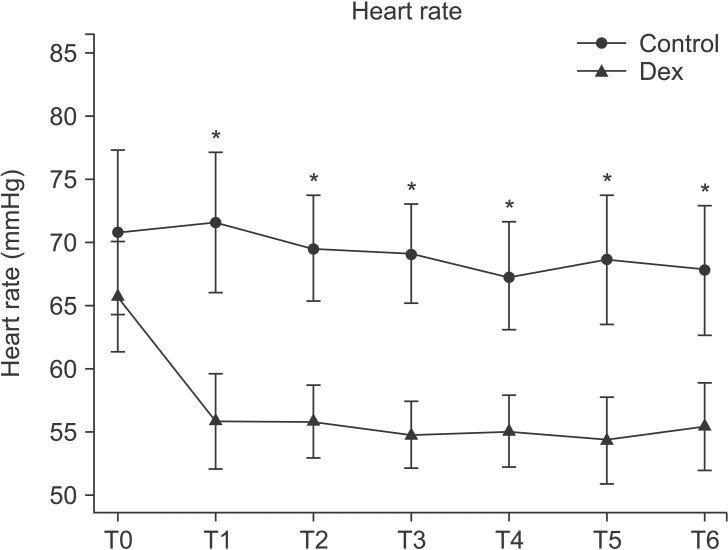
Table 1
Patients' Characteristics
Table 2
Patients' Sedation Score Using the Ramsay Sedation Scale
|
Dexmedetomidine group (n = 22) |
Control group (n = 20) |
|
|---|---|---|
| Deepest sedation scale (%) | ||
| 1-2 | 0 (0%) | 20.0 (100%) |
| 3-4 | 13.0 (59.1%) | 0 (0%) |
| 5-6 | 9.0 (40.9%) | 0 (0%) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download