Eisenmenger's syndrome (ES) is considered as the most severe form of pulmonary arterial hypertension related to congenital cardiac anomalies [1]. Due to high maternal mortality rate in these patients (30-50%) [2], termination of pregnancy is generally recommended. However, in the case of continuing gestation, a multidisciplinary approach with cardiologists, cardiac surgeons, obstetricians, and anesthesiologists should be required for the safe pregnancy and delivery [3].
A 27-year-old patient of multigravida (height 164 cm, weight 71 kg) with known ES was scheduled for cesarean section. When she was pregnant at the first time, she terminated pregnancy according to cardiologist's advice. However, after 4 months, she revisited the hospital at 8 weeks of gestation and refused the second termination of pregnancy. At 26 weeks of gestation, she was admitted for the management of worsening pulmonary arterial hypertension and heart failure.
On physical examination, heart rate was 88 bpm and blood pressure was 100/51 mmHg. Oxygen saturation was maintained between 88 and 92%. Transthoracic echocardiography showed complicated atrioventricular septal defect with bidirectional shunt, severe pulmonary hypertension (mean pulmonary artery pressure of 66 mmHg measured by maximum velocity of pulmonary regurgitation), dilated right ventricle with mildly depressed right ventricular systolic function, right ventricular hypertrophy, right atrial enlargement, and preserved left ventricular function (ejection fraction of 68%). With O2 supplement using facial mask, the continuous infusion of treprostinil (10 ng/kg/min) was started. At 32 weeks of gestation, non-sustained ventricular tachycardia (NSVT) was frequently developed. After discussing about the optimal managements with a cardiologist, an obstetrician, and an anesthesiologist, an emergent cesarean section under general anesthesia was decided. Extracorporeal membrane oxygenation was prepared in case of developing biventricular dysfunction before starting anesthesia.
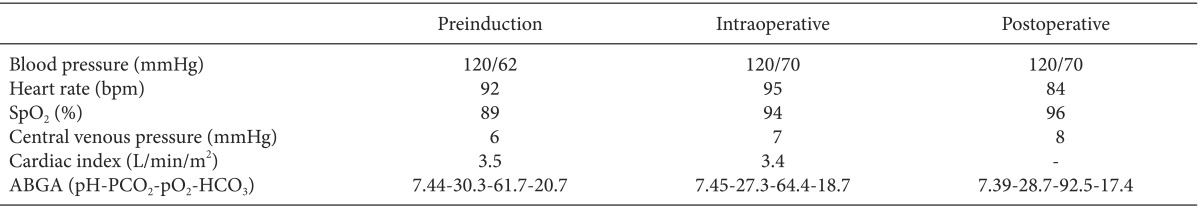
On the arrival at the operating room, 5-lead electrocardiography, non-invasive blood pressure monitoring, and pulse oxymetry were applied on the patient, and continuous infusion of amiodarone was initiated at 20 mg/hr to prevent NSVT. After applying the elastic stockings for the prophylaxis of deep vein thrombosis, a radial artery catheter and a right internal jugular venous catheter were placed before inducing anesthesia. A dedicated transducer (FloTrac, Edwards Lifesciences, Irvine, CA, USA) was connected to the radial arterial line on one side and to the Vigileo system (Edwards Lifesciences, Irvine, CA, USA) on the other side for the continuous monitoring of cardiac output. The pre-induction blood pressure, central venous pressure, oxygen saturation, and cardiac index were 120/62 mmHg, 6 mmHg, 89%, and 3.5 L/min/m2, respectively.
After preoxygenation, anesthesia was induced using 2 mg of midazolam, 10 mg of etomidate, and continuous infusion of remifentanil with cricoid pressure. Neuromuscular block was achieved using 70 mg of rocuronium. Transesophageal echocardiography (TEE) was then inserted. There was no significant change compared to preoperative echocardiography finding. Mechanical ventilation was set with a tidal volume of 450 ml and an inspired oxygen fraction of 0.5. The respiratory rate was adjusted to maintain the pressure of end-tidal carbon dioxide at 30 to 35 mmHg. Mean airway pressure and oxygen saturation were maintained at 20 cmH2O and at 93 to 94%, respectively. Anesthesia was maintained with a continuous infusion of propofol and remifentanil to prevent the increasing of PVR so that the target bispectral index was between 40 and 60. A live male baby at 1.84 kg with Apgar score of 4 at 1 minute and 8 at 5 minutes was extracted and transferred to neonatal intensive care unit. During the whole course of anesthesia, acidosis, hypercarbia, and hypoxemia which require corrections did not occur, and no hemodynamic instability developed (Table 1).
After surgery, the patient was transferred to an intensive care unit with endotracheal tube and was taken to the cardiologist. Postoperative pain was managed with intravenously infused opioid. Anticoagulation with heparin was started from postoperative day (POD) 1 and was continued for the next 1 week. Extubation was performed on POD 1. She was transferred to the general ward on POD 10. On POD 14, she was discharged without any specific complications.
In a normal pregnancy, plasma volume increases and the systemic and pulmonary vascular resistances decrease. However, in patients with ES, an irreversibly increased pulmonary vascular resistance restricts blood flow to the lungs. Therefore, an increased plasma volume adds burden to the already compromised right ventricle [3,4]. Moreover, systemic vasodilation with pre-existing pulmonary hypertension increases the right-to-left shunt, which worsens the hypoxia [3,4]. These series of changes occur in a vicious cycle of increases in the pulmonary hypertension, right ventricular strain, and right-to-left shunt [3].
In this case, we chose the method of general anesthesia, due to the patient's request and for the monitoring of intraoperative TEE. Unfortunately, the conduction of anesthesia can increase the right-to-left shunt in these patients, regardless of the choice between general and regional anesthesia. Therefore, maintaining the stable hemodynamics is more important than the choice of anesthesia.
The use of appropriate invasive monitoring devices such as direct arterial catheter, central venous catheter, pulmonary arterial catheter, and TEE might be helpful [5]. However, we did not use pulmonary arterial catheter for several reasons. Firstly, cardiac output by thermodilution technique would be unpredictable in the presence of large intracardiac shunt. Secondly, the risk of pulmonary artery rupture would be high. Lastly, arrhythmia induced by the catheter could be risky in the case of our patient. Instead, we chose Vigileo monitor (Vigileo/FloTrac, Edwards Lifesciences, Irvine, CA, USA) for the continuous monitoring of cardiac output. In addition, we did our best to avoid the conditions that would increase pulmonary arterial resistance (i.e., hypothermia, acidosis, hypercarbia, and hypoxemia).
We did not use preoperative anticoagulation. An obstetrician was concerned about the postoperative bleeding. Considering that any hypovolemia could result in a sudden death in the case of our patient [4], our team decided to follow the obstetrician's suggestion.
The postoperative pain could also increase the systemic vascular resistance. Therefore, we decided to wake the patient up after achieving the appropriate postoperative analgesia.
In conclusion, maintaining the balance between systemic vascular resistance and pulmonary vascular resistance without myocardial depression would be the key to safe anesthetic management in a pregnant patient with ES.
References
1. Kaemmerer H, Mebus S, Schulze-Neick I, Eicken A, Trindade PT, Hager A, et al. The adult patient with eisenmenger syndrome: a medical update after dana point part I: epidemiology, clinical aspects and diagnostic options. Curr Cardiol Rev. 2010; 6:343–355. PMID: 22043211.

2. Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998; 31:1650–1657. PMID: 9626847.

3. Minicucci S, Segala V, Verdecchia C, Sismondi P, Casabona R, Sansone F. Safe management of cesarean section in a patient of Eisenmenger syndrome. Ann Card Anaesth. 2012; 15:296–298. PMID: 23041687.

4. Borges VT, Magalhães CG, Martins AM, Matsubara BB. Eisenmenger syndrome in pregnancy. Arq Bras Cardiol. 2008; 90:e39–e40. PMID: 18516394.
5. Martin JT, Tautz TJ, Antognini JF. Safety of regional anesthesia in Eisenmenger's syndrome. Reg Anesth Pain Med. 2002; 27:509–513. PMID: 12373701.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download