This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The impact of volatile induction using large-dose sevoflurane (VI-S) on cerebral blood flow has not been well investigated. The present study compared the changes in cerebral blood flow of middle cerebral artery using transcranial Doppler (TCD) during VI-S and conventional induction using propofol.
Methods
Patients undergoing elective lumbar discectomy were randomly allocated to receive either sevoflurane (8%, Group VI-S, n = 11) or target-controlled infusion of propofol (effect site concentration, 3.0 µg/ml; Group P, n = 11) for induction of anesthesia. The following data were recorded before and at 1, 2, and 3 min after commencement of anesthetic induction (T0, T1, T2, and T3, respectively): mean velocity of the middle cerebral artery (VMCA) by TCD, mean blood pressure (MBP), heart rate, bispectral index score (BIS) and end-tidal CO2 (ETCO2). Changes in VMCA and MBP from their values at T0 (ΔVMCA and ΔMBP) at T1, T2, and T3 were also determined.
Results
BISs at T1, T2 and T3 were significantly less than that at T0 in both groups (P < 0.05). ΔVMCA in Group VI-S at T2 and T3 (18.1% and 12.4%, respectively) were significantly greater than those in Group P (-7.6% and -19.8%, P = 0.006 and P < 0.001, respectively), whereas ETCO2 and ΔMBP showed no significant intergroup difference.
Conclusions
VI-S using large-dose sevoflurane increases cerebral blood flow resulting in luxury cerebral flow-metabolism mismatch, while conventional propofol induction maintains cerebral flow-metabolism coupling. This mismatch in VI-S may have to be considered in clinical application of VI-S.
Keywords: Cerebral blood flow, Sevoflurane, Volatile induction and maintenance of anesthesia
Introduction
Volatile induction and maintenance of anesthesia using sevoflurane has been regarded as an ideal combination because of the favorable characteristics of sevoflurane, such as its nonpungent smell and low blood : gas partition coefficient. Although volatile induction using large-dose sevoflurane (VI-S) is applied selectively in patients who have no intravenous access, its application can provide rapid and satisfactory anesthetic induction comparable to intravenous induction with propofol.
VI-S, without any adjuvant anesthetic agents, employs much larger dosage of sevoflurane to hasten anesthetic induction: the dose of sevoflurane should be abruptly increased to reach more than 4-8 vol% during a very brief period. Many previous studies have speculated about sevoflurane's inconsistent effects on cerebral blood flow (CBF) during anesthesia maintenance, which vary from reductions to increases in flow depending on its dose in various settings [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. However, the effect of VI-S with sevoflurane > 4% on CBF has not been well examined during the very beginning of the anesthesia in the clinical setting.
We hypothesized that VI-S may increase CBF and that the degree of CBF increase would be greater than that during conventional intravenous induction using propofol boluses. To examine this, we analyzed changes in blood flow velocity in the middle cerebral artery (MCA) using transcranial Doppler (TCD) during the application of VI-S, and compared this and other parameters against data collected during anesthetic induction using propofol in healthy patients undergoing general anesthesia.
Materials and Methods
Our Institutional Review Board approved the study protocol, and written informed consent was obtained from each patient prior to enrollment in the study. The enrolled patients were confined to those with an American Society of Anesthesiologists physical status of I and Mallampati classification of 1, and all underwent one- or two-level lumbar discectomy. An equal number of recruited patients were randomly allocated to undergo either VI-S (Group VI-S) or intravenous induction using propofol (Group P) for anesthetic induction; allocation was performed using assignment cards with identification numbers concealed within non-transparent envelopes that were shuffled and handed out during the preoperative anesthesia visit.
Patients allocated to Group VI-S were instructed regarding the following steps of anesthetic induction procedures until they were mastered: maximal exhalation, inhalation of 8 vol% sevoflurane-primed oxygen through a facemask, and repetition of maximal inspiration and expiration to the best of their ability.
Exclusion criteria were as follows: current treatment with opioids, active respiratory disease, cardiovascular disease (e.g., hypertension), diabetes, neurological disease, gastroesophageal reflux, heavy snoring, myopathy, recent head injury, or unfavorable airway for mask ventilation.
Upon arrival at the patient holding area, a 20 G cannula was placed in the radial artery after infiltration of local anesthetics to perform continuous invasive blood pressure (BP) and initial arterial CO2 tension (PaCO2) monitoring just before anesthetic induction. To avoid hypotensive episodes during anesthetic induction, 10 ml/kg of lactated Ringer's solution and 0.2 mg of glycopyrrolate were administered through an 18 G intravenous line placed in the right forearm vein. Monitoring included pulse oximetry, electrocardiography, and the bispectral index (BIS) upon arrival of the patient in the operation room.
In the first stage of our study, a 2-MHz TCD probe (100M™ monitor with FlowTrax™ M-mode Doppler; Spencer Technologies, Seattle, WA, USA) was fitted against the participant's cranium, and the probe's location and angle were adjusted by a well-trained and experienced physician's assistant who was blinded to the study to achieve a sufficient TCD profile of the MCA at a depth of 45 to 55 mm. A headband with a specially designed frame was used to secure the position of the probe and to maintain a constant insonation angle throughout the study period. Automated baseline tracing of the time-averaged mean velocity of the MCA (VMCA), mean BP (MBP), heart rate (HR), and PaCO2 were determined and recorded by the physician's assistant immediately before anesthetic induction (T0).
In the second stage, anesthesia was induced as follows for each of the treatment groups. In Group VI-S, the patient's end of the breathing circuit was closed and the breathing circuit, CO2 absorber, and reservoir bag were primed with 8% sevoflurane in oxygen flowing for 2 min at 9 L/min using an anesthesia delivery unit (Aestiva/5™; GE Healthcare, Madison, WI, USA). After priming, a transparent facemask was applied and the patient was required to exhale maximally through the mask and then inhale oxygen (9 L/min) with 8% sevoflurane primed in the respiratory circuit and reservoir bag. The patient repeated the deepest exhalation and inhalation possible upon the attending anesthesiologist's request. During this period, they were asked to abduct the left arm at a 45° angle and to maintain that position for as long as possible. With loss of arm tone or a decline in the patient's respiratory effort, the anesthesiologist applied assisted or controlled ventilation to maintain an ETCO2 of 30 to 35 mmHg. After reaching an expiratory sevoflurane concentration of 4 vol%, the inspired sevoflurane concentration was reduced to maintain an expiratory concentration of around 4 vol%.
In Group P, the patients were asked to abduct the left arm at a 45° angle and to maintain that position for as long as possible. They were then administered an intravenous target-controlled propofol infusion with an effect-site concentration of 3.0 µg/ml. With loss of arm tone or a decline in the patient's respiratory effort, the anesthesiologist applied assisted or controlled ventilation of oxygen (9 L/min) through a facemask to maintain an ETCO2 of 30 to 35 mmHg.
Rocuronium (0.7-0.9 mg/kg) was intravenously administered to facilitate tracheal intubation at the decline of the BIS index, and then train-of-four monitoring at 20 s-intervals was initiated in both groups. The trachea was intubated at the abolition of the train-of-four response.
Transient hypotension was treated with leg elevation and additional intravenous administration of 200 to 300 ml of 6% hydroxethyl starch (Voluven™; Fresenius-Kabi, Bad Homburg, Germany). MBP was maintained within 30% of the baseline value at T0. Each patient's body temperature was maintained at 36 to 37℃ using a forced-air warming device throughout the entire anesthetic procedure.
Patients in whom the following conditions occurred were excluded: displacement of the TCD probe resulting in loss of insonation; hypotension (MBP reduction of > 30% compared to T0 or MBP of < 60 mmHg) requiring the use of a vasoconstrictor; coughing; incomplete exhalation or breath-holding; and insufficient assisted or controlled ventilation during loss of spontaneous respiration. Patients in whom the serial TCD procedure for successful assessment of MCA insonation was not possible during the entire study period were also excluded from the study.
Data and statistical analysis
All data, including MBP, HR, ETCO2, and VMCA, were recorded before anesthetic induction (T0) and at 1, 2, and 3 min after induction (T1, T2, and T3, respectively). In addition, changes in VMCA and MBP at T1, T2, and T3 relative to T0 (ΔVMCA and ΔMBP) were determined. First, the normality of data distributions was evaluated using the Kolmogorov-Smirnov test. Next, normally distributed data were compared using a parametric test, and abnormally distributed data were compared using a nonparametric test. Intergroup comparisons of MBP, HR, ETCO2, and VMCA at T0 were performed with the t-test or Mann-Whitney U-test, as appropriate.
Because VMCA, ΔVMCA, ΔMBP and BIS values were non-normally distributed, Friedman's repeated-measures analysis of variance (ANOVA) by rank was used. MBP, HR, and ETCO2 data were normally distributed and passed the sphericity assumption (Mauchly's sphericity test). Repeated-measures ANOVA was used to analyze their values, followed by a post-hoc Bonferroni test for multiple comparisons.
The moving cross-correlation between MBP and VMCA were analyzed by Pearson correlation to determine the impact of each induction on cerebral autoregulation during the study period.
The data were analyzed using the Statistical Package for the Social Sciences (SPSS® for Windows, version 18.0; SPSS, Chicago, IL, USA). In all analyses, P < 0.05 was considered statistically significant.
The primary outcome in this study was VMCA at T0, T1, T2, and T3. To determine the required sample size, annual TCD tracing data for the MCA of our institution had been evaluated. The mean ± standard deviation for VMCA in the propofol induction group was 34.9 ± 9.2 cm/sec, and autocorrelation between adjacent measurements on the same subject was 0.8.
For our power calculation, we assumed first-order autocorrelation. We wanted to detect a 30% difference in VMCA of different groups and a 30% difference at different time points. Therefore, the minimum number of patients per group was 11 with an α of 0.05 and a power of 80%. If a recruited patient was excluded during the study, the subsequent patient took over the identification number of the previously excluded patient.
Results
Patients were randomly and serially recruited to each group until appropriate TCD data had been gathered for 22 patients (11 patients in each group). Three of 25 recruited patients were excluded from the study because of involuntary movement or coughing that resulted in a loss of proper TCD insonation; thus, the data of 22 patients were included in the statistical analysis (
Table 1).
Table 2 shows the values of V
MCA, MBP, PaCO
2, ETCO
2, HR and BIS at T0, T1, T2, and T3.
VMCA and MBP at T0, T1, T2, and T3 did not show intragroup differences in Group VI-S, whereas VMCA and MBP at T3 were significantly lower than those at T0 in Group P (all P = 0.012). PaCO2 and HR did not show intergroup differences. ETCO2 at T1 in Group VI-S was significantly lower than PaCO2 at T0 in Group VI-S and ETCO2 at T1 in Group P (all P ≤ 0.001). HR at T2 was significantly higher than that at T0 in Group P (P < 0.05). BIS values at T1, T2 and T3 were significantly lower than those at T0 in both groups (P < 0.001), and BIS values at T1 were significantly greater in VI-S compared with those in Group P (P < 0.001).
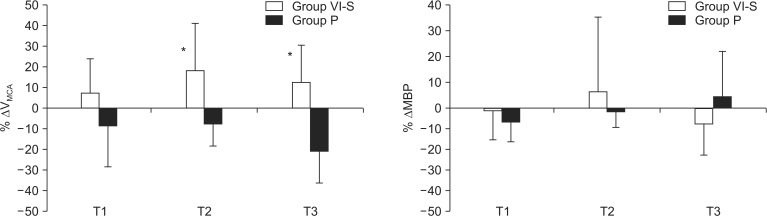
With respect to the primary outcome (
Fig. 1), ΔV
MCA values at T2 (18.1%, +22.6 cm/sec) and T3 (12.4%, +18.1 cm/sec) were significantly greater for Group VI-S than corresponding ΔV
MCA values observed for Group P at T2 (-7.6%, -10.1 cm/sec, P = 0.006) and T3 (-19.8%, -14.8 cm/sec, P < 0.001). ΔMBP at T1, T2, and T3 did not show intergroup differences (P = 0.780, 0.830, and 0.063, respectively).
Pearson's coefficients between MBP and VMCA in Group VI-S and Group P were -0.381 (P < 0.001) and 0.120 (P = 0.289, respectively).
Finally, the number of patients requiring leg elevation due to transient reduction of ΔMBP > 30% from the MBP at T0 did not show a significant intergroup difference (3/11 in Group VI-S, 7/11 in Group P, P = 0.086).
Discussion
This study examined changes in CBF and its major determinants including MBP, end-tidal CO2 and cerebral activity (BIS values) during VI-S in a clinical setting, and it compared outcomes against those observed during conventional anesthetic induction using intravenous propofol. CBF during VI-S, as determined by changes in VMCA (ΔVMCA), increased as the patient moved from wakefulness (T0) to several minutes after induction (18.1 and 12.4% increases at T2 and T3 from the baseline value, respectively), whereas conventional propofol induction reduced VMCA from the baseline values.
Although previous work has shown that sevoflurane reduces cerebral activity in a dose-dependent manner, the overall impacts of sevoflurane on CBF were variable and dependent on the dose. Sevoflurane at a modest dose (0.5-1.5 minimum alveolar concentration [MAC], < 1.25 MAC for a BIS of 40-50, < 1.0 MAC for a BIS of 55) reduced CBF proportionally to the decline in cerebral activity or provided a constant CBF regardless of any change in aortic blood flow by maintaining cerebral autoregulation [
1,
2,
3,
4,
5,
6,
7,
8,
9]. A commonly used dose of sevoflurane for a surgical level of anesthesia (MAC 0.7-0.9 for a BIS of 45-55) produced vasodilation in large cerebral vessels and vasoconstriction in resistance arterioles, along with a decline in cerebral metabolism [
10]. In contrast, most previous studies using sevoflurane at a much larger dose (dose for a BIS of 35 or 4-8 inspired vol% for EEG burst-suppression) showed dose-dependent increases in CBF and attenuated cerebral autoregulation, resulting in luxury cerebral perfusion with reduced cerebral activity [
11,
12,
13]. The results of the present study correspond well with these previous results regarding increases in CBF with the use of large-dose sevoflurane.
The possible contributions of the intra- or intergroup differences in cerebral perfusion pressure to the increases in CBF during VI-S were excluded by maintaining a constant MBP during the entire VI-S procedure, which was confirmed through the absence of any significant intergroup differences in ΔMBP.
However, considering previous study showing that an already increased CBF due to the use of large-dose sevoflurane did not induce further increase in CBF [
3], we excluded the possible contribution of the greater PaCO
2 into the greater CBF during the initial VI-S. Therefore, we concluded that the primary cause of increased CBF during sevoflurane induction, as indicated by increased V
MCA, was cerebral arterial vasodilation due to the use of a larger dose of sevoflurane > 4.0 vol%. This is supported by previous research into sevoflurane's sympatholytic properties, which showed that this drug indirectly attenuates sympathetic nervous modulation of the vascular system and creates a vasodilating effect [
14,
15]. The negative correlation coefficient between MBP and V
MCA in Group VI-S contrast to the lacking of correlation in Group P may support compromise in cerebral autoregulation during VI-S contrast to its preservation during propofol induction.
The present study also confirmed that both VI-S and conventional propofol induction reduces cerebral activity. Previous studies also showed the equipotent dosage of sevoflurane for achieving a BIS of 40 were 1.5-1.7 end-tidal vol%, which were comparable to propofol 3.7-4.1 µg/ml, respectively [
2,
3,
16].
Meanwhile, Gupta et al. [
5] also showed in which large-dose sevoflurane induces cerebral vasodilation resulting in luxury flow-metabolism uncoupling, contrast to the constant reduction in V
MCA in the use of propofol along with the decline of cerebral activity resulting in preserved cerebral flow-metabolism coupling: sevoflurane 1.7 end-tidal vol% for achieving a BIS 50 from awake status reduced V
MCA (by 24%) but further increment to end-tidal 2-4 vol% for achieving a BIS 35 paradoxically increased V
MCA (by 18%). Of note, they employed 15 min interval during dose-increment for achieving stable alveolar-cerebral equilibrium, which was not applicable to the present study. Interestingly, VI-S requires at least 1-2 min for achieving BIS < 60, while propofol-induction provides faster decline of BIS < 60 within 1 min in the present study. The greater mean BIS value at T1 in Group VI-S (65 vs. 39 in Group P) and the less number of patients who achieved BIS values < 60 at T1 in Group VI-S (5/11 vs. 11/11 patients in Group P) also support the longer duration for achieving the same level of hypnosis in VI-S. This delay in VI-S seems to be due to the lag for achieving alveolar-blood-cerebral equilibrium of sevoflurane concentration. Considering this aspect, the actual end-tidal sevoflurane concentration may be far less than the larger-dose of sevoflurane, as the initial 8 inspired vol% and the subsequent 5-6 inspired vol% during the initial 1-2 min of the VI-S procedure. In the present study, the end-tidal concentration of sevoflurane showed considerable fluctuation probably due to the larger tidal volume and hold of breath and it was unable to be determined.
Changes in CBF are closely related with V
MCA and are proportional to the degree of V
MCA change in previous studies employing TCD [
17,
18,
19]. However, the actual amount (volume) of CBF cannot be directly measured using TCD; instead, the CBF change can be inferred by determining the degree of V
MCA change: if the arterial diameter is constant or not reduced, the degree of CBF increase will be proportional to increments in V
MCA. By contrast, vasoconstriction without increasing cerebral blood volume also increases blood flow velocity: any condition that reduces the arterial diameter compromises the efficacy of TCD for a reliable determination or estimation of the change in CBF by misinterpreting the exaggerated velocity as an increased CBF. Therefore, we did not use a vasoconstrictor to compensate for a sudden or transient decline in BP during the anesthesia induction period; instead, pre-anesthetic volume loading and elevation of the patient's leg were employed to maintain the intravascular volume status or to redistribute the intravascular volume during this study period.
Because the aim of this study was to compare the two types of practical anesthesia induction, we attempted to replicate typical VI-S procedure by instructing patients to take a couple of deep breaths and briefly hold their breath. As a result, despite our effort to maintain normocarbia using controlled and assisted ventilation during the entire induction procedure, ETCO
2 at 1 min after the start of the VI-S procedure (T1) was significantly lower than that in Group P. This might raise concerns about a possible association of hypocarbia and resultant vasoconstriction of the MCA. However, a previous study showed that in contrast to medium-sized cerebral arteries, the diameter of large cerebral arteries, such as the MCA, is not affected by even considerable change in BP or ETCO
2 [
20]. On the contrary, breath-holding immediately after taking in a couple of large tidal volumes may interfere with the equilibrium between ETCO
2 and PaCO
2, resulting in underestimation of the ETCO
2 during this period.
The observed effects of VI-S with respect to cerebral vasodilation and aggravated cerebral autoregulation, resulting in luxury perfusion due to cerebral flow-metabolism mismatch, must be considered before its application as an anesthetic induction technique. Although the vasodilatory properties of VI-S may be beneficial in terms of avoiding cerebral vasoconstriction or spasm, the decoupling of CBF and metabolism during luxury perfusion can be problematic in patients who are vulnerable to any increase in CBF.
In conclusion, we showed that VI-S increased CBF (12-18%) but conventional propofol induction reduced CBF (8-20%) in adult patients. Considering the consistent reduction in cerebral activity in both methods, this result suggests that large-dose sevoflurane for VI-S produces a direct vasodilatory effect on cerebral arteries resulting in luxury cerebral flow-metabolism mismatch, while conventional propofol induction maintains cerebral flow-metabolism coupling. This discrepancy and luxury mismatch during VI-S may have to be considered in the choice of anesthesia induction methods.
Acknowledgments
This study was supported by Konkuk University Medical Center, Konkuk University School of Medicine.
References
1. Holzer A, Greher M, Hetz H, Standhardt H, Donner A, Heinzl H, et al. Influence of aortic blood flow velocity on changes of middle cerebral artery blood flow velocity during isoflurane and sevoflurane anaesthesia. Eur J Anaesthesiol. 2001; 18:238–244. PMID:
11350461.

2. Kaisti KK, Långsjö JW, Aalto S, Oikonen V, Sipilä H, Teräs M, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003; 99:603–613. PMID:
12960544.
3. Holzer A, Winter W, Greher M, Reddy M, Stark J, Donner A, et al. A comparison of propofol and sevoflurane anaesthesia: effects on aortic blood flow velocity and middle cerebral artery blood flow velocity. Anaesthesia. 2003; 58:217–222. PMID:
12603451.

4. Hinohara H, Kadoi Y, Takahashi K, Saito S, Goto F. Differential effects of propofol on cerebrovascular carbon dioxide reactivity in elderly versus young subjects. J Clin Anesth. 2005; 17:85–90. PMID:
15809122.

5. Gupta S, Heath K, Matta BF. Effect of incremental doses of sevoflurane on cerebral pressure autoregulation in humans. Br J Anaesth. 1997; 79:469–472. PMID:
9389265.

6. Summors AC, Gupta AK, Matta BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesth Analg. 1999; 88:341–345. PMID:
9972753.
7. Matta BF, Mayberg TS, Lam AM. Direct cerebrovasodilatory effects of halothane, isoflurane, and desflurane during propofol-induced isoelectric electroencephalogram in humans. Anesthesiology. 1995; 83:980–985. PMID:
7486184.

8. Cho S, Fujigaki T, Uchiyama Y, Fukusaki M, Shibata O, Sumikawa K. Effects of sevoflurane with and without nitrous oxide on human cerebral circulation. Transcranial Doppler study. Anesthesiology. 1996; 85:755–760. PMID:
8873545.
9. Schlünzen L, Vafaee MS, Cold GE, Rasmussen M, Nielsen JF, Gjedde A. Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand. 2004; 48:1268–1276. PMID:
15504187.

10. Molnár C, Settakis G, Sárkány P, Kálmán S, Szabó S, Fülesdi B. Effect of sevoflurane on cerebral blood flow and cerebrovascular resistance at surgical level of anaesthesia: a transcranial Doppler study. Eur J Anaesthesiol. 2007; 24:179–184. PMID:
16970835.

11. Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999; 91:677–680. PMID:
10485778.

12. Reinsfelt B, Westerlind A, Ricksten SE. The effects of sevoflurane on cerebral blood flow autoregulation and flow metabolism coupling during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2011; 55:118–123. PMID:
21039354.
13. Conti A, Iacopino DG, Fodale V, Micalizzi S, Penna O, Santamaria LB. Cerebral haemodynamic changes during propofol-remifentanil or sevoflurane anaesthesia: transcranial Doppler study under bispectral index monitoring. Br J Anaesth. 2006; 97:333–339. PMID:
16829673.

14. Ogawa Y, Iwasaki K, Shibata S, Kato J, Ogawa S, Oi Y. The effect of sevoflurane on dynamic cerebral blood flow autoregulation assessed by spectral and transfer function analysis. Anesth Analg. 2006; 102:552–559. PMID:
16428560.

15. Schlünzen L, Juul N, Hansen KV, Gjedde A, Cold GE. Regional cerebral glucose metabolism during sevoflurane anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol Scand. 2010; 54:603–609. PMID:
20085540.
16. Schlünzen L, Juul N, Hansen KV, Cold GE. Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol Scand. 2012; 56:248–255. PMID:
22091956.

17. Dahl A, Lindegaard KF, Russell D, Nyberg-Hansen R, Rootwelt K, Sorteberg W, et al. A comparison of transcranial Doppler and cerebral blood flow studies to assess cerebral vasoreactivity. Stroke. 1992; 23:15–19. PMID:
1731414.
18. Kochs E, Hoffman WE, Werner C, Albrecht RF, Schulte am. Cerebral blood flow velocity in relation to cerebral blood flow, cerebral metabolic rate for oxygen, and electroencephalogram analysis during isoflurane anesthesia in dogs. Anesth Analg. 1993; 76:1222–1226. PMID:
8498657.

19. Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994; 25:793–797. PMID:
7909175.

20. Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993; 32:737–741. PMID:
8492848.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download