Abstract
The trigemino-cardiac reflex has been reported to occur during various craniofacial surgeries or procedures including manipulation of the trigeminal ganglion, tumor resection in the cerebellopontine angle, various facial reconstructions and trans-sphenoidal adenomectomy. Regarding risk factors during trans-sphenoidal adenomectomy, invasiveness closely related to the size of tumor and the degree of manipulation of cavernous sinus wall have been reported. We report the case of a 40-year-old female patient who had a relatively small-sized (< 10 mm) pituitary adenoma. Repetitive asystoles occurred during microscopic trans-sphenoidal operation of the wall of the cavernous sinus, which strongly suggests the importance of careful manipulation of the cavernous sinus wall. In addition to reporting this rare complication of trans-sphenoidal adenomectomy, we reviewed its clinical management by performing a literature search.
The term trigemino-cardiac reflex (TCR) was used after more generalized involvement of all facial and head areas that are innervated by trigeminal nerves was observed; and the previously known phenomenon, oculocardiac reflex, was replaced by the term 'trigemino-cardiac reflex' [1]. The clinical features of TCR vary from bradycardia, asystole following bradycardia, to asystole without preceding bradycardia and arterial hypotension [2,3]. The TCR occurs due to stimulation of the peripheral or central part of the trigeminal nerve, such as when manipulating the temporomandibular joint, zygomatic bone, orbital cavity, pituitary fossa, or cranial nerve (V, VIII) exit areas near the brainstem. With regard to trans-sphenoidal adenomectomy, the TCR occurs during extraction of the pituitary adenoma from the cavernous sinus or while preparing the nasal septum [2,3]. The tumor size and invasiveness were identified as the precipitating factors that are related to the status of the tumor [3,4]. Here we report a case of asystole that occurred during the operation of a relatively small-sized (< 10 mm) pituitary adenoma as a result of aggressive manipulation near the cavernous sinus, thus highlighting the importance of careful manipulation of the cavernous sinus wall.
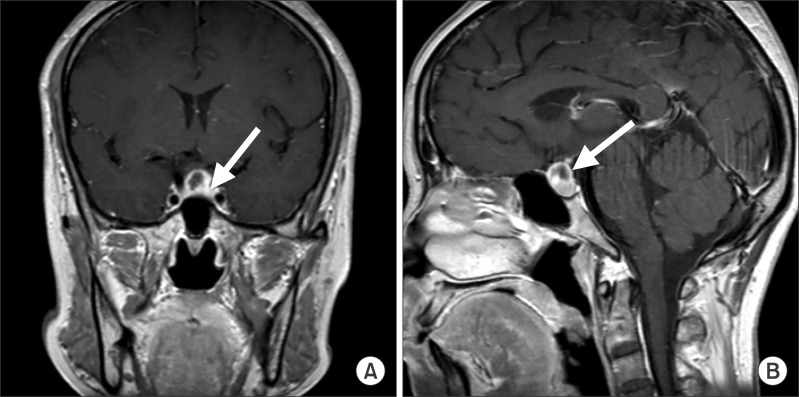
A 40-year-old female patient (159 cm, 56 kg) was admitted for elective trans-sphenoidal adenomectomy for the treatment of a prolactin-secreting pituitary adenoma. Her chief complaint was headache, and her visual field examination was normal. Although the prolactin level was 393 ng/ml (4-30 ng/ml), there were no related symptoms. Magnetic resonance imaging (MRI) showed the presence of a small pituitary adenoma (9 × 10 mm) with focal hemorrhage within the sella (Fig. 1). She had no history of cardiovascular disease and there were no abnormalities on the electrocardiogram (ECG) and chest X-ray. Following routine monitoring and preoxygenation, anesthesia was induced with intravenous propofol and remifentanil with a target controlled infusion pump (Orchestra® Base Primea, Fresenius Kabi, Brezins, France) followed by rocuronium. The target effect-site concentrations for propofol and remifentanil during the induction were 4 µg/ml and 4 ng/ml, respectively. Invasive arterial blood pressure line was made in the left radial artery. Vital signs were stable from the time of induction up to the period before the event, and blood pressure (BP) was 110-130/60-70 mmHg and heart rate (HR) was 60-70 /min.
After approximately 5 hours, a 0.2 cm frozen tissue sample and two 0.4 × 0.4 cm tissue samples were harvested by surgical biopsy from the central portion of the tumor. However, hemorrhagic tissue with no evidence of a pituitary adenoma was found in the frozen section and hence the surgeon decided to further explore the medial wall of the left cavernous sinus (Fig. 1. arrowed area). During further manipulation with a curette to obtain a sample for an additional frozen section biopsy, the patient developed sudden severe bradycardia (20-30 /min) accompanied by a decrease in the BP to 80/35 mmHg for approximately 2 seconds. After this, asystole was noted for approximately 10 seconds on the ECG, and concurrently no waves were seen on the arterial pressure monitor. The surgical procedure was stopped immediately. After this, the normal sinus rhythm was restored following transient supraventricular dysrhythmia without the use of anticholinergic or any other medications. At the time of initial bradycardia, remifentanil and propofol were infused at target concentrations of 3 ng/ml and 3.5 µg/ml, respectively. During this episode, laboratory test values were as follows: pH 7.44, PaCO2 36 mmHg, PaO2 281 mmHg (FiO2 0.55), potassium 3.1 mmol/L, and ionized calcium 0.84 mmol/L. The BP and HR were restored to 105-110/55-60 mmHg and 65-70 /min, respectively.
When the surgery was resumed, leakage of cerebrospinal fluid was detected near the left cavernous sinus wall, suggesting the rupture of the arachnoid membrane due to aggressive curettage. The surgeon decided that further procedure should be stopped and only dural repair should be performed. After thirty minutes, during the dural repair procedure, asystole recurred for about 4 seconds as soon as the manipulation near the cavernous sinus wall was started. The surgical procedure was stopped again. During this second episode, remifentanil and propofol were infused at target effect site concentrations of 2.6 ng/ml and 2.8 µg/ml, respectively. Normal sinus rhythm was restored again and the dural repair was restarted and completed without occurrence of any further episodes of bradycardia or asystole. The surgical time was 6 hours 25 minutes, and the anesthesia time was 7 hours 30 minutes. 5,600 ml of fluid was infused, including 500 ml of colloids. Two units of packed RBCs were transfused.
The patient was transferred to the intensive care unit (ICU). In the ICU, the BP and HR were stable within the range of 100-120/50-60 mmHg and 70-85 /min, respectively. No arrhythmia was displayed on the ECG. Until this time, the diagnosis of trigemino-cardiac reflex was not suspected. To identify the cause of asystole, 2-D echocardiography was performed; and it did not show any regional wall motion abnormality and the ejection fraction was 61%. Also 24-hour Holter monitoring was performed, but there was no occurrence of significant arrhythmias. Histo-pathologic examination confirmed the diagnosis of a pituitary adenoma with hemorrhagic infarction. Through discussion and literature search, we arrived at the diagnosis of trigeminocardiac reflex. The patient was transferred to the ward on the next day and discharged from the hospital 3 days later without any complications.
It has been reported that sudden bradycardia or asystole develops during various surgical procedures or instrumentation in the perioperative period [5,6]. Among them, the conditions that develop due to the stimulation of the areas supplied by branches of the trigeminal nerve are considered under the term TCR [1,2]. TCR can be defined as the occurrence of bradycardia or asystole that is triggered by surgical or various procedural stimuli to the areas innervated by trigeminal afferents (V1, V2, and V3). The mechanistic process underlying the development of clinical features involves the neural circuits that connect the trigeminal nucleus and vagal motor nucleus within the brainstem and the final vagal efferent fibers to the heart [2,7]. There are no well-controlled, prospective studies evaluating the risk factors for this reflex, and this is probably owing to the rarity and unpredictable nature of this condition.
Schaller [3] performed a retrospective analysis by comparing two groups of patients with or without the occurrence of the TCR during endoscopic trans-sphenoidal surgery. The two groups showed a significant difference in the degree of tumor invasiveness into the parasellar tissue, but not in the overall size of the tumor. The insignificant difference in the tumor size seems to be related to the fact that the author compared the longest diameters of the tumors regardless of the direction of tumor growth. In another retrospective study [4] of 1,040 trans-sphenoidal surgeries that were performed in 17 years, only 3 cases of TCR were reported; and this finding again emphasizes the causal relationship between the manipulation of cavernous sinus wall and TCR. In this latter study, all 3 cases showed a large sized mass with lateral expansion into the cavernous sinus. Regarding the tumor in our case, it was a relatively small sized tumor; however, it was suspicious for invasion into the cavernous sinus wall. When the surgeon tried to remove some tissue from the cavernous sinus wall, the TCR occurred repeatedly; thereby suggesting the causal relationship between this procedure and TCR.
There are known risk factors for the development of TCR such as the use of opioids, calcium channel blockers, β adrenergic receptor blockers, hypoxia, hypercarbia, light general anesthesia, previous heart disease, etc [6,8]. Although these risk factors have been evaluated for the oculocardiac reflex, they have also been applied to the more general form of the condition, the trigemino-cardiac reflex [1,6,8]. However in our case, we could not identify any evidence of hypercarbia, hypoxia, use of high-risk medications or any other heart disease. Also the level of anesthesia was adequate, considering that the blood pressure and heart rate were stable. The authors suppose that the reason why the TCR occurred in this case even though these risk factors were not present lies in the many possible ways of modulating the occurrence of the reflex, especially at the level of the brainstem [7,9].
By studying the effects of remifentanil on the occurrence of oculocardiac reflex, Chung et al. [10] showed a greater decrease in heart rate compared with that after the use of sevoflurane (23% vs 11%). This result suggests the possible role of remifentanil as a risk factor for the occurrence of TCR; however, the dose (0.5 µg/kg/min) was much higher than that in our case (0.1 µg/kg/min, Orchestra®). Other studies, using small doses of rapid-acting opioids, report the potentiating effects of opioids on the occurrence of TCR [11,12]. Hence, the possibility of potentiating effects of opioids in our case cannot be excluded. Therefore, if the anesthesiologist wants to use opioids during trans-sphenoidal surgery, he should be more cautious about the occurrence of TCR.
The majority of reports state that patients with bradycardia or asystole recover after discontinuing the manipulation or administration of anticholinergics like atropine or glycopyrrolate [1,3]. But some reports state that the use of epinephrine, a temporary pacemaker or a cardiac massage was needed upon the failure of atropine to treat the TCR [6,13,14,15]. The dose of epinephrine varies in the reports from 5 µg [13] to 1 mg [6,14,15]. Therefore in cases with the TCR, the use of epinephrine, a pacemaker or a cardiac massage should be considered upon the failure of atropine.
Regarding the preventive measures for TCR, a regional nerve block or an intravenous atropine injection have shown successful results [1,3,6]. But there are many case reports that show that an intramuscular injection of glycopyrrolate as a premedication has no preventive effect on the occurrence of TCR [2,13]. Another important point to be considered for the prevention of TCR is the knowledge of high-risk surgical procedures that are performed around the head and face [2,6] or the knowledge of a known high-risk area like the cavernous sinus [3,4]. Before high-risk procedures are performed, there should be a consensus about the possible occurrence of TCR between the surgeon and the anesthesiologist. Also, the surgeon should be aware that an abrupt, strong and prolonged stimulation can cause TCR [2,8].
In conclusion, the authors report that the manipulation of cavernous sinus wall during trans-sphenoidal surgery should be carried out with extreme caution regardless of the tumor size. The anesthesiologist should be able to detect the occurrence of TCR early and warn the surgeon accordingly. Also, surgeons should know that atropine usually has a good effect on reversing the TCR, but the possibility of non-response should also be kept in mind.
References
1. Bohluli B, Ashtiani AK, Khayampoor A, Sadr-Eshkevari P. Trigeminocardiac reflex: a MaxFax literature review. Oral Surg Oral Med Oral pathol Oral Radiol Endod. 2009; 108:184–188. PMID: 19615657.

2. Schaller B, Cornelius JF, Prabhakar H, Koerbel A, Gnanalingham K, Sandu N, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009; 21:187–195. PMID: 19542994.
3. Schaller B. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clin Neurol Neurosurg. 2005; 107:468–474. PMID: 16202819.

4. Cho JM, Min KT, Kim EH, Oh MC, Kim SH. Sudden asystole due to trigemino-cardiac reflex during trans-sphenoidal surgery for pituitary tumor. World Neurosurg. 2011; 76:477.e11–477.e15. PMID: 22152579.

5. Doyle DJ, Mark PW. Reflex bradycardia during surgery. Can J Anaesth. 1990; 37:219–222. PMID: 2088315.

6. Lübbers HT, Zweifel D, Grätz KW, Kruse A. Classification of potential risk factors for trigeminocardiac reflex in craniomaxillofacial surgery. J Oral Maxillofac Surg. 2010; 68:1317–1321. PMID: 20347202.

7. Schaller B. Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? J Neurol. 2004; 251:658–665. PMID: 15311339.
8. Blanc VF, Hardy JF, Milot J, Jacob JL. The oculocardiac reflex: a graphic and statistical analysis in infants and children. Can Anaesth Soc J. 1983; 30:360–369. PMID: 6871777.

9. Wang X, Gorini C, Sharp D, Bateman R, Mendelowitz D. Anaesthetics differentially modulate the trigeminocardiac reflex excitatory synaptic pathway in the brainstem. J Physiol. 2011; 589:5431–5442. PMID: 21930602.

10. Chung CJ, Lee JM, Choi SR, Lee SC, Lee JH. Effect of remifentanil on oculocardiac reflex in paediatric strabismus surgery. Acta Anaesthesiol Scand. 2008; 52:1273–1277. PMID: 18823468.

11. Arnold RW, Jensen PA, Kovtoun TA, Maurer SA, Schultz JA. The profound augmentation of the oculocardiac reflex by fast acting opioids. Binocul Vis Strabismus Q. 2004; 19:215–222. PMID: 15530138.
12. Campbell R, Rodrigo C, Cheung L. Asystole and bradycardia during maxillofacial surgery. Anesth Prog. 1994; 41:13–16. PMID: 8629742.
13. Prabhakar H, Ali Z, Rath GP. Trigemino-cardiac reflex may be refractory to conventional management in adults. Acta Neurochir (Wien). 2008; 150:509–510. PMID: 18465091.

14. Jaiswal AK, Gupta D, Verma N, Behari S. Trigeminocardiac reflex: a cause of sudden asystole during cerebellopontine angle surgery. J Clin Neurosci. 2010; 17:641–644. PMID: 20188568.

15. Hemmer LB, Afifi S, Koht A. Trigeminocardiac reflex in the Postanesthesia care unit. J Clin Anesth. 2010; 22:205–208. PMID: 20400008.

Fig. 1
T1-weighted enhanced MRI of the sella showing a pituitary tumor (< 10 mm) which has a cystic portion, presumably the hemorrhagic necrotic area. (A) coronal view, (B) sagittal view. The arrow indicates the suspicious tumor invasion area, prompting the surgeon to obtain more tissue for performing a frozen section biopsy.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download