Abstract
Antiphospholipid syndrome (APS) is a rare disease in which patients display prolonged coagulation test results in vitro, but usually develop thrombotic symptoms in vivo. Patients with APS are at increased risk of valvular heart disease or coronary vascular disease, conditions that often necessitate cardiac surgery via bypass. The management of anticoagulation during cardiopulmonary bypass (CPB) is particularly challenging in these patients because of the unique features of APS. Patients with APS are constantly at risk of arterial and venous thrombotic events. Therefore it is very important to maintain proper anticoagulation perioperatively, especially during CPB. In this paper, we present three successful cases of APS patients who underwent cardiac surgery with CPB.
Antiphospholipid syndrome (APS) is a paradoxical disease that shows a coagulation defect on some coagulation tests but that causes thrombotic disease in vivo [1]. The frequency of APS is 1-5% in young, healthy people and 30% in patients with systemic lupus erythematosus (SLE) [2]. The disease causes venous, arterial, and small vessel thrombosis, pregnancy loss, and preterm delivery for patients with severe pre-eclampsia or placental insufficiency. Other clinical manifestations include cardiac valvular disease, renal thrombotic microangiopathy, thrombocytopenia, hemolytic anemia, and cognitive impairment. The term catastrophic APS is used to define an accelerated form of APS resulting in multiorgan failure [3]. It has been reported that catastrophic APS may occur in surgical cases even when the diagnosis is known preoperatively. In such cases, the mortality rate is nearly 50% despite the patients receiving intensive care. Because patients with APS display a coagulation delay in laboratory tests but actually show a thrombotic tendency in vivo, it is very challenging situation when patients with APS is scheduled to undergo cardiac surgery with cardiopulmonary bypass (CPB). Massoudy et al. reported that out of a population of 5,706 patients who underwent CPB, five were diagnosed with APS. Of these five, three expired during the perioperative period, and only one remained alive a year after the surgery [4]. Adequate anticoagulation both during CPB and during weaning from CPB are important prognostic factors for APS patients. It has not yet been determined, however, which coagulation test is the best for patients undergoing CPB, or how to interpret its results. The authors encountered three APS patients who underwent cardiac surgery with CPB and who did not develop major complications either during or after the operation, and we present their cases in the following paper.
A 23-year-old man (192 cm, 106.4 kg) presented to the authors for pulmonary thromboembolism and was scheduled for pulmonary artery thrombectomy. He has been diagnosed with APS after the treatment of deep vein thrombosis in his right leg at another hospital four years earlier, and he had been prescribed an anticoagulant medicine. Two weeks before the patient's hospital admission, he stopped taking the medication at his discretion. Tenderness, edema, and skin ulcers occurred in both his lower extremities. During his hospital stay, the patient developed dyspnea, and pulmonary thromboembolism was diagnosed in both his descending pulmonary arteries and lower lobe segmental and subsegmental arteries via echocardiography and chest computed tomography. The result of the lung perfusion scan that was performed was also suitable for pulmonary thromboembolism. The preoperative diluted Russell's viper venom time (dRVVT) result was 112.80 s (reference: 24-37.5 s), and the silica clotting time (SCT) was prolonged to 185.6 s (reference: 23.4-39.2 s). Arterial oxygen tension was 70 mmHg under room air. The mixing test was not corrected, and the result was 112.80 s with the patient plasma, and 73.3 s with the mixed plasma. The patient was thus diagnosed with lupus anticoagulant (LA). The anticardiolipin antibodies (ACAs) IgG, and IgM were positive, the antithrombin III was 85% of the normal level, the protein C was reduced to 66%, and the protein S was reduced to 57%. Enoxaparine 120 mg was injected via the subcutaneous tissue twice a day and prednisolone was administered during the preoperative period. An abnormal coagulation profile was observed; prothrombin time/international normalized ratio (PT/INR) was 1.9, prothrombin time (PT) was 22.3 s and activated partial thromboplastin time (aPTT) was 79.1 s. Preoperative estimated pulmonary arterial systolic pressure (PASP) was 76 mmHg.
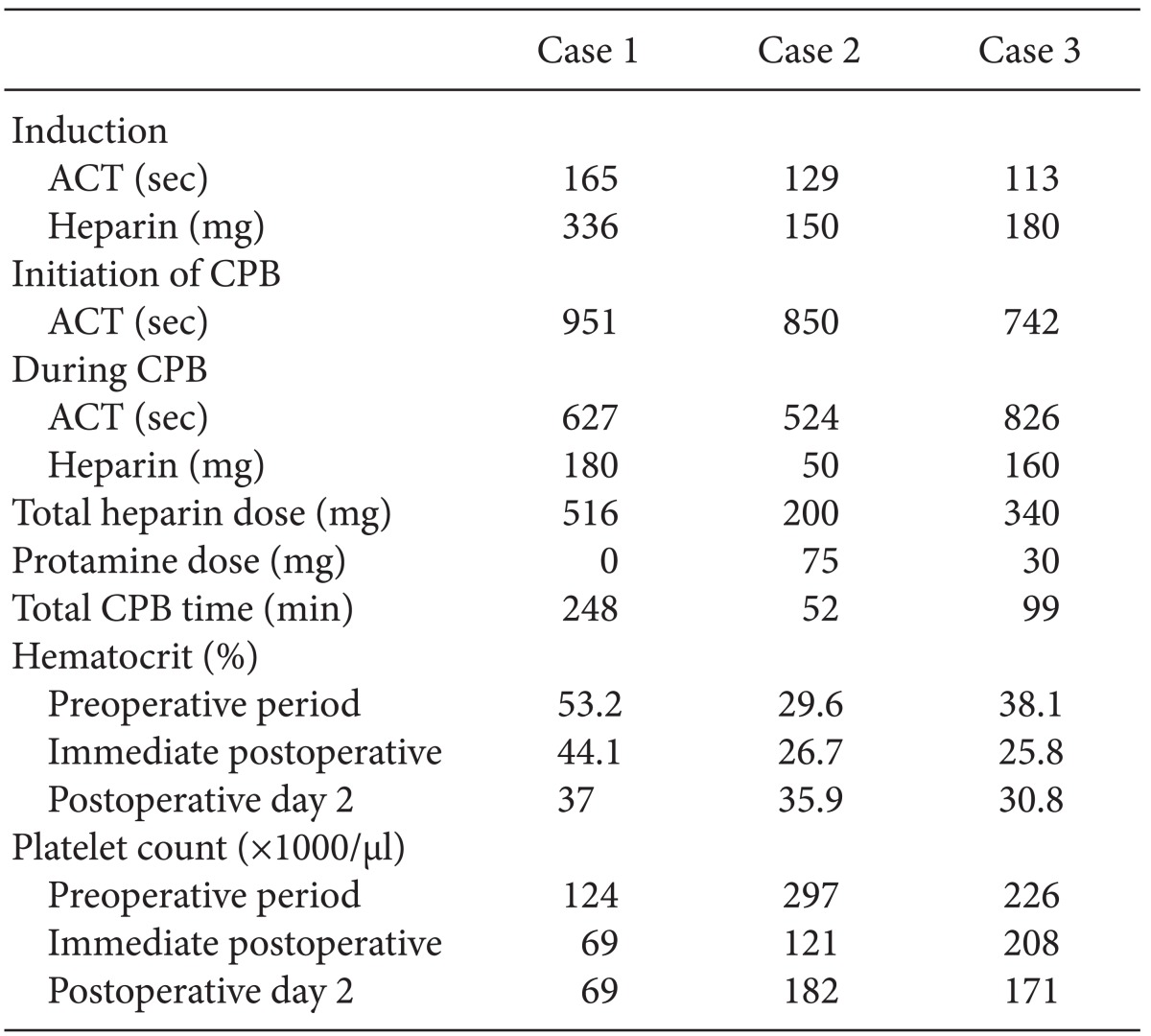
Radial artery catheterization was performed, and midazolam (13 mg), sufentanil (150 µg), and vecuronium (10 mg) were injected for induction of anesthesia. The routine monitoring devices for cardiac surgery and intravenous anesthetics were used for maintenance of anesthesia. Initial point-of-care testing (POCT) results were the following: arterial oxygen tension was 201 mmHg under a 50% fraction of inspired oxygen (FiO2), and plasma hematocrit was 54%. The activated coagulation time (ACT) was 165 s after induction and 951 s after injection of 336 mg of heparin to begin CPB. CPB was started after insertion of the cannulas into the aorta, superior vena cava, and inferior vena cava. After the temperature was lowered to 20℃, pulmonary thrombectomy and endarterectomy were performed, following which intermittent total cardiopulmonary bypass using aortic-cross-clamp was conducted to clear the operating field. Forty minutes after the administration of heparin, the ACT was checked again and found to be 627 s. Generally, such a result would be considered acceptable for a CPB operation, but concerns about the occurrence of catastrophic APS in this case, prompted the empirical administration of 100 mg of heparin via injection. The ACT was again measured 5 min after the injection and was found to be 1200 s. After 3 hours, the ACT was found to be 629 s. An additional 80 mg of heparin was thus administered, and the ACT was found to be 1001 s 5 min after that injection. It was decided that protamine would not be administered due to worries about the occurrence of postoperative thrombotic complications. Forty minutes after the last heparin injection, weaning from CPB was successfully performed. The total injected dose of heparin was 516 mg, and the total CPB time was 248 min. At the end of the surgery, POCT was again performed. Arterial oxygen tension was 332 mmHg under 50% FiO2 and plasma hematocrit was 42%. The estimated blood loss (EBL) during surgery was 1,000 ml and urine output was 1,100 ml. 1,500 ml of crystalloid and 1,400 ml of colloid were infused during surgery. No transfusion was performed during the surgery.
Following the procedure the patient was transferred to the intensive care unit (ICU) without experiencing significant bleeding. The immediate postoperative PT/INR was 1.63 and aPTT was over 180 s. The goal of the postoperative anticoagulation therapy was PT/INR 3.0 with intravenous heparin infusion during the NPO period and with oral warfarin after that. PASP was not normalized after the surgery; postoperative PASP was 74 mmHg. The patient was discharged 12 days after surgery without specific complications.
A 34-year-old man (160.5 cm, 48.9 kg) was presented to the authors for mitral valve (MV) replacement. The patient had been diagnosed with SLE and APS after being admitted to the hospital for treatment of palpitations and dyspnea ten years earlier, and he had taken an anticoagulant medication. During the outpatient clinic follow-up, 24-hr Holter monitoring revealed the increasing frequency of non-sustained atrial fibrillation. An operation was thus scheduled to reduce the risk of atrial thrombosis. The preoperative blood coagulation tests showed prolonged results. The aPTT was 57.8 s and dRVVT was 47.2 s (reference: 25.9-35.7 s). The result with patient plasma from the mixing test was 27.3 s, and the mixed plasma result was 37.6 s, while SCT was 52.1 s (reference: 22.3-42.7 s). In addition, the patient was positive for LA, negative for ACA IgG, and had borderline results for ACA IgM. Intravenous heparin and oral deflazacort tablets were used for preoperative management.
Invasive blood pressure monitoring was conducted via the patient's radial artery. Anesthesia induction and maintenance were performed as usual, and routine cardiac anesthesia monitoring was done. The ACT level increased from 129 s to 850 s after the injection of 150 mg of heparin. After the insertion of cannulas into the aorta, superior vena cava, and inferior vena cava, CPB was started. One hour after the administration of heparin, the ACT level was found to have decreased to 524 s. Therefore, an additional 50 mg of heparin was injected. After the mitral annuloplasty, valvuloplasty was performed, and weaning from CPB was attempted. Due to the risk of thrombosis, a half dose (75 mg) of protamine was administered. Five minutes after the injection of protamine, the ACT level was found to be 220 s. The total administered dose of heparin during the surgery was 200 mg, and the total CPB time was 52 min. The EBL during surgery was 500 ml and urine output was 455 ml. 800 ml of crystalloid and 500 ml of colloid were infused during surgery. The patient did not receive any transfusion during the surgery.
Following the surgery the patient was transferred to the ICU without significant bleeding. The immediate postoperative PT/INR was 1.39 and aPTT was over 180 s. Postoperative prophylactic anticoagulation was maintained with warfarin. Ten days after the operation, the patient was discharged without specific complications.
A 65-year-old woman (153 cm, 62.9 kg) presented to the authors for MV leaflet mass excision. She already had percutaneous mitral valvuloplasty 10 years ago, MV replacement with tissue valve 8 years ago, and MV thrombectomy and MV replacement with mechanical valve 7 years ago. Since the final surgery, she had been on an anticoagulation regimen with warfarin. Recently she had gone to emergency room after experiencing sudden onset language disturbance, and had been diagnosed with left middle cerebral artery territory infarction. She also underwent routine follow-up transesophageal echocardiography (TEE) and a mobile mass was found which was attached to the prosthetic MV leaflet. To prevent an embolic event and decrease the size of the thrombus, the patient was started on heparin several days before the surgery was scheduled. Despite heparinization, however, the thrombus showed no change in size on TEE. The surgeon therefore considered that the patient might have a disease causing a hypercoagulable state and ordered laboratory examinations to investigate this possibility. The results of the tests were as follows: Protein C (-), protein S (-), antithrombin III 80 (80-120%), ACA IgM 15.5 mg/dl (<20), ACA IgG negative, LA (+), dRVVT 69.1 s (reference: 24-37.5 s), SCT 137.2 (reference: 23.4-39.2 s), mixing test with patient plasma 137.2 s, mixing test with mixed plasma (1 : 1) 99.3 s. Twelve weeks later, the LA test was performed again, and the patient was finally diagnosed with APS. Preoperative PT/INR was 1.37 and aPTT was 59.8 s.
The planned surgery was MV leaflet mass excision. The patient's left radial artery was cannulated under local anesthesia for invasive blood pressure monitoring. Routine induction and maintenance of anesthesia with IV anesthetics were performed. The central venous catheter was accessed and a TEE probe was inserted. The patient's basal ACT was 113 s and 180 mg of heparin was administered for vessel cannulations. The ACT level increased to 742 s and CPB was begun after insertion of cannulas into the aorta, superior vena cava and inferior vena cava. Twenty minutes after the start of CPB, the ACT level was found to be 826 s, and an additional 60 mg of heparin was administered. The target ACT level during CPB was over 800 s. After 50 min, the ACT level had decreased to 682 s, and another 100 mg of heparin was given. At the end of CPB, the ACT level was 668 s, and the surgeon decided not to administer protamine, concerned about possible postoperative thrombotic complications. One hour after the cessation of CPB, the ACT level decreased to 577 s. The total time of CPB was 99 min, and the total administered dose of heparin was 340 mg. The EBL during surgery was 600 ml and the urine output was 2,710 ml. 200 ml of crystalloid and 2,000 ml of colloid were infused during surgery. No transfusion was administered during the surgery.
Following the procedure, the patient was transferred to the ICU. The immediate postoperative PT/INR was 2.93 and aPTT was over 400 s. Due to a bleeding tendency, 30 mg of protamine was administered to the patient. With that exception, there were no other specific complications. Warfarin was used for prophylactic anticoagulation and the patient was discharged 9 days after the operation.
The three case mentioned above are summarized in Table 1.
According to the recently revised guidelines for APS the criteria for definite APS include one or more episodes of thrombosis, a symptom related to thrombosis, and a positive antiphospholipid antibody test (aPL test) result on two or more occasions that are at least 12 weeks apart [5]. The reason for such a time interval requirement is that about 4-5% of healthy individuals can present positive results temporarily due to infections or drugs taken [2]. Two important autoimmune antigens related to the pathophysiologic mechanism of APS that have been discussed are β-2-glycoprotein-1 (β2GPI) and prothrombin. The coupling of β2GPI and the autoimmune antibody induces blood coagulation in vivo through the signal-transporting system of the cell, but in vitro, the coagulation time is delayed by the addition or formation of negatively charged phospholipids (PL(-)). Prothrombin (coagulation factor II) also forms prothrombin/antiprothrombin complex, which binds with PL(-), delaying the coagulation time in vitro. The formation or addition of PL(-) triggered coagulation time delay in the test (aPTT and dRVVT). As the aPL in the patient plasma bound with PL(-) in the normal plasma, the delay of the coagulation test was not corrected in the mixed-coagulation test. The PT is thus not useful in the detection of aPL because excessive phospholipids in the form of tissue thromboplastin are added.
The therapeutic goal in patients with APS is the prevention of thrombosis. There are two different prophylactic settings: primary thromboprophylaxis for patients who have not yet experienced a thrombotic event, and secondary thromboprophylaxis for patients who have already had a previous thrombotic event. For primary prophylaxis, patients are prescrived low dose aspirin, and for secondary prophylaxis, patients take warfarin with target PT/INR 2.0 to 3.0 [6].
APS patients are more vulnerable than the general public to cardiac valvular disease because if endocardial injury (mainly on the left side of the heart) due to the pressure or jet of blood flow occurs, the PL(-) in the cell are exposed, which induces microthrombi [7]. The valve injury thus progresses due to the fibrotic change of the microthrombi. Among the aPL antibodies, the autoimmune antibody to β2GPI is frequently detected in paitents with chest pain syndrome or those who are undergoing acute coronary events. Such patients may undergo surgery using CPB, which makes the selection of an appropriate coagulation test or method particularly important.
In APS patients, several factors must be considered to be able to choose which coagulation test to perform or which laboratory values to use as the guidelines for the anticoagulation therapy. This is the case because the coagulation test results can be misinterpreted in APS and because the use of CPB can induce an immunologic and a coagulative response. In addition, the destruction of blood cells produces a large amount of PL(-). There has thus far been no consensus regarding which is the best method of anticoagulation is, and only a handful of cases that seem to point in the direction of any particular method have been reported. In some studies, either the standard dose (3 mg/kg) or an increased dose (5 mg/kg) of heparin was used regardless of the ACT [8]. Sheikh et al. [9] recommended a heparin dose that doubles the baseline ACT level. The use of the ACT as means to measure blood coagulation is accompanied by a number of problems, namely: (1) there may be patients with increased baseline ACT levels; (2) half of APS patients may have thrombocytopenia; and (3) the heparin response may vary due to a patient's low level of antithrombin III. In the cases discussed here, all three of our patients' baseline ACT levels were in the normal range. In addition, adequate prolongations of ACT levels were achieved with the standard dosage of heparin in our patients. Ducart et al. [10] maintained a plasma heparin level of over 2.5 IU/ml. In some cases, the heparin-ACT response curve was drawn preoperatively. This method focused on the heparin concentration rather than on its effect. Measurement of the antifactor Xa level has been recently introduced, but there is yet no conclusion as regards which method is most effective and leads to the fewest complications. Adequate diagnostic tools and guidelines must be established according to the hospital circumstances as well as the patient's individual risk factors, and the optimal anticoagulation effect that does not increase the bleeding must be maintained. Moreover, the neutralization of heparin with protamine after the completion of CPB should be carefully done. In some reports, protamine was not used to prevent catastrophic APS [4,11]. In the first case discussed here, although the ACT level was prolonged during both the operation and the weaning from CPB, a standard dose of heparin was administered regardless of the ACT result, and protamine was not injected during weaning from CPB due to worries about the occurrence of severe complications, such as catastrophic APS. In the second case, half the standard dose of protamine was administered empirically. And in the third case, a minimal dose of protamine (30 mg) was administered in the ICU postoperatively due to a bleeding tendency. In such cases, the surgical procedure can be completed simply through the use of any of the above methods, but considering the risk of bleeding, it is reasonable to determine the appropriate dose through the continuous infusion of protamine (for example 50 mg/hr [10]) until the dose no longer causes bleeding.
Catastrophic APS is an extremely serious complication that may occur during the postoperative period. The incidence and mortality of catastrophic APS are about 1% and 50%, respectively. The syndrome's exact cause has not yet been determined, but it is believed that operations and infections may trigger it. To prevent this, infections should be controlled through administration of appropriate antibiotics, and a parenteral anticoagulant should be taken by patients who will undergo surgery peripartum and by patients with rashes associated with SLE. It has been reported that heparin administration to maintain the PT/INR level at about 3, and steroid use to prevent the release of cytokine from necrotic tissue, may help prevent infection [12].
Taking all these factors into consideration, the anesthesiologist should use caution in interpreting the results of the coagulation panel and in preventing thrombosis during the perioperative period. Further studies are also necessary for the maintenance and monitoring of an adequate anticoagulation level, especially during CPB in APS patients.
References
1. Esmon NL, Safa O, Smirnov MD, Esmon CT. Antiphospholipid antibodies and the protein C pathway. J Autoimmun. 2000; 15:221–225. PMID: 10968914.

2. Shi W, Krilis SA, Chong BH, Gordon S, Chesterman CN. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust N Z J Med. 1990; 20:231–236. PMID: 2115326.

3. Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003; 12:530–534. PMID: 12892393.
4. Massoudy P, Cetin SM, Thielmann M, Kienbaum P, Piotrowski JA, Marggraf G, et al. Antiphospholipid syndrome in cardiac surgery - an underestimated coagulation disorder? Eur J Cardiothorac Surg. 2005; 28:133–137. PMID: 15982596.
5. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4:295–306. PMID: 16420554.

6. Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010; 376:1498–1509. PMID: 20822807.

7. Hojnik M, George J, Ziporen L, Shoenfeld Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996; 93:1579–1587. PMID: 8608627.

8. Myers GJ, Hirsch GM. Double valve replacement in a patient with anticardiolipin antibody syndrome. Perfusion. 1999; 14:397–401. PMID: 10499657.

9. Sheikh F, Lechowicz A, Setlur R, Rauch A, Dunn H. Recognition and management of patients with antiphospholipid antibody syndrome undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1997; 11:764–766. PMID: 9327321.
10. Ducart AR, Collard EL, Osselaer JC, Broka SM, Eucher PM, Joucken KL. Management of anticoagulation during cardiopulmonary bypass in a patient with a circulating lupus anticoagulant. J Cardiothorac Vasc Anesth. 1997; 11:878–879. PMID: 9412889.

11. Dornan RI. Acute postoperative biventricular failure associated with antiphospholipid antibody syndrome. Br J Anaesth. 2004; 92:748–754. PMID: 15003982.

12. Cervera R, Bucciarelli S, Plasín MA, Gómez-Puerta JA, Plaza J, Pons-Estel G, et al. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the "CAPS Registry". J Autoimmun. 2009; 32:240–245. PMID: 19324520.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download