Abstract
A precise pre-procedural evaluation of mitral valve (MV) pathology is essential for planning the surgical strategy for severe mitral regurgitation (MR) and preparing for the intraoperative procedure. In the present case, a 38-year-old woman was scheduled to undergo MV replacement due to severe MR. She had a history of undergoing percutaneous balloon valvuloplasty due to rheumatic mitral stenosis during a previous pregnancy. A preoperative transthoracic echocardiography suggested a tear in the mid tip of the anterior mitral leaflet. However, the "en face" view of the MV in the left atrial perspective using intraoperative real time three-dimensional transesophageal echocardiography (RT 3D-TEE) provided a different diagnosis: a torn cleft in the P2-scallop of the posterior mitral leaflet (PML) with rupture of the chordae. Thus, surgical planning was changed intraoperatively to MV repair (MVRep) consisting of patch closure of the PML, commissurotomy, and lifting annuloplasty. The present case shows that intraoperative RT 3D-TEE provides more precise and reliable spatial information of MV for MVRep and facilitates critical surgical decision-making.
Because mitral regurgitation (MR) is a complex disorder resulting from structural abnormalities in the mitral valve (MV) apparatus, a pre-procedural evaluation of MV and a precise diagnosis are necessary for planning surgical intervention of MR and determining a postoperative prognosis. Two-dimensional (2D) transthoracic echocardiography (TTE) provides a sufficient preoperative diagnosis for MR pathology [1]. However, due to the structural complexity of the MV apparatus and the limited ability of 2D echocardiography to delineate cardiac structures, sufficient information for an evaluation and precise diagnosis cannot be provided without adopting additional imaging or diagnostic modalities.
In the present case, intraoperative real time three-dimensional transesophageal echocardiography (RT 3D-TEE) provided a more precise diagnosis for the main MR pathology, which was greatly different from the preoperative diagnosis suggested by preoperative 2D TTE. Thus, the predetermined surgical strategy employing MV replacement (MVR) was changed to MV repair (MVRep).
A 38-year-old woman planned to undergo open heart surgery to surgically correct a known severe MR. She had a history of undergoing percutaneous balloon valvuloplasty due to rheumatic mitral stenosis (MS) during a previous pregnancy 10 years before. At the preoperative evaluation employing 2D TTE, a tearing of the tip of a mid part of the thickened anterior mitral valve leaflet (AML) was suggested to be the main reason for a coaptation defect producing severe MR; two-chambered views using color Doppler showed a severe central MR jet projecting into an enlarged left atrium (Fig. 1, Video 1 and 2). MVR to install a bioprosthetic valve was planned preoperatively considering the technical difficulties and worse outcome of a patch repair of a large defect on the free margin of the AML.
After induction of anesthesia, a 3D-TEE probe (X7-2t transducer; Philips Healthcare, Andover, MA, USA) was placed and connected to a TEE console (iE33™, Philips Healthcare, Andover, MA, USA) and intraoperative 2D and 3D TEE examinations were performed. The 2D TEE images in the midesophageal commissural and long axis views also supported the preoperative diagnosis regarding a severe MR and systolic coaptation defect between the AML and posterior mitral leaflet (PML) (Fig. 2, Video 3 and 4). However, intraoperative real time 3D TEE provided more detailed spatial information on the MR pathology, which was entirely different from that suggested by the preoperative TTE; the "en face view" in the left atrial perspective showed a cleft in the middle of the PML, a ruptured chordae attached to the P3 scallop moving toward the left atrium during systole, and fusion of both ends of the MV commissures producing mild MS (Fig. 3, Video 5). Interestingly, the defect or cleft in the AML, which had been suggested, was not observed.
The feasibility of MVRep was reevaluated considering this new and different intraoperative diagnosis. Finally, after confirming a cleft in the P2 scallop by direct observation through atriotomy, MVRep, consisting of posterior mitral valvuloplasty with patch closure and lifting posterior annuloplasty, was successfully performed.
Two-dimensional TTE and TEE provide spatial information that facilitates a thorough understanding of objective cardiac structure, pre-procedural surgical planning, and intraoperative decision-making during surgical intervention for severe MR. Regardless of the surgeon's expertise and experience, a thorough and detailed evaluation to determine the feasibility of MVRep is important during surgical intervention for severe MR, considering the better survival of MVRep, low incidence of endocarditis, better preservation of heart function, low risk of stroke and infection, and the lack of a need for long-term anticoagulant use [2,3]. Furthermore, thorough and detailed structural information is beneficial to determine the level of procedural difficulties during MVRep.
A previous report showed that intraoperative 2D TEE accurately provides MV anatomy and predicts the suitability of MVRep [1]. Independent predictors of unsuccessful MVRep include a central jet, annular calcification or severe dilatation, and extensive leaflet disease. However, due to the limitation of 2D echocardiography for delineating cardiac structures in a single 2D image, it requires multiple well-aligned 2D images to create a virtual 3D cardiac structure in the brain, and considerable effort to align the image planes. Indeed, 2D TEE and TTE require a long period of training to acquire adequate skill and knowledge for appropriate and comprehensive 2D imaging and interpretation. Furthermore, acquiring appropriate 2D images for a full evaluation of the corresponding pathology can be difficult for complex pathologies, as shown in the present case. Even the combination of preoperative 2D TTE and intraoperative TEE may not provide sufficient 2D imaging to enable a thorough understanding of MR pathology and to make a precise diagnosis. In this circumstance, 3D echocardiography or other imaging modalities are useful for evaluating the full spectrum of concurrent pathology.
As in the present case, RT 3D TEE is particularly helpful for evaluating cardiac structures located near the aligned TEE probe (perpendicular to the sonographic beam), such as MV and LA [4]; a single "en face" image from RT 3D TEE depicts the true anatomic perspective of the MV apparatus and facilitates an understanding of MR pathology in a much easier and faster manner [5,6]. After making this new diagnosis, MVRep employing PML patching was performed instead of MVR, which was the preoperatively determined surgical plan.
Although chorda repair or transfer for AML prolapse is easily and successfully performed in most cases, repairing the defect or damaged free margin of AML is much more complex and challenging, even to experienced surgeons [7], compared to techniques for repairing of PML [8]. The thickened and solid part in a longer and wider AML can hinder its homogenous motion after applying autologous or synthetic materials. This may lead to post-procedural re-intervention or a switch to MVR due to a residual or newly developed MR. Furthermore, the feasibility and success rate of MVRep in patients with rheumatic valve disease is much lower than that in patients with degenerative MV disease (about 75% vs. 95%) [2,9]. This discrepancy is probably due to a varying degree of inflammatory change in the rheumatic MV structure with extensive fibrosis and calcification of the leaflet free margin and chordal fusion, which compromises the success or outcome of MVRep. Therefore, surgeons prefer MVR to MVRep (patching) for a torn AML margin in a patient with rheumatic MV disease, as in the present case. However, despite the technical difficulties and relatively lower success rate of MVRep in rheumatic valve disease, it is recommended and preferred to MVR as a primary surgical strategy [10-12] considering that the success rate of MVRep is 75% [9].
A surgeon would be able to confirm the MV pathology and perform a similar surgical process in the absence of a new and different pre-procedural diagnosis upon intraoperative RT 3D TEE. However, the importance of a prompt pre-procedural diagnosis cannot be overemphasized considering the possible intraoperative delay to confirm the discrepancy in the diagnosis, check the feasibility of MVRep to modify the surgical strategy, and prepare surgical devices or materials.
In conclusion, the present case demonstrates the clinical efficacy of intraoperative RT 3D TEE for enabling a thorough and precise pre-procedural evaluation of severe MR and its pathophysiology and facilitating intraoperative decision-making.
Notes
References
1. Omran AS, Woo A, David TE, Feindel CM, Rakowski H, Siu SC. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J Am Soc Echocardiogr. 2002; 15:950–957. PMID: 12221412.

2. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008; 52:e1–142. PMID: 18848134.
3. Di Bardino DJ, El Bardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg. 2010; 139:76–84. PMID: 19931098.
4. Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, Bednarz JE, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr. 2006; 19:413–421. PMID: 16581480.

5. Wilens HJ, Ferrer PL, Tamer DF, Labrador E, Aqatston AS, Keith K, et al. Cor triatriatum sinister in an adult: management guided by real time three-dimensional transesophageal echocardiography and stress echocardiography. Echocardiography. 2010; 27:E132–E136. PMID: 20553320.
6. Biaggi P, Jedrzkiewicz S, Gruner C, Meineri M, Karski J, Vegas A, et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J Am Soc Echocardiogr. 2012; 25:758–765. PMID: 22537396.

7. Sulcaj L, Rizza A, Glauber M, Trianni G, Palmieri C, Ravani M, et al. Influence of involvement of anterior leaflet versus posterior leaflet on residual regurgitation as assessed by transesophageal echocardiography in patients undergoing valve repair for mitral regurgitation due to mitral valve prolapse. Cardiovasc Ultrasound. 2009; 7:54. PMID: 19922602.

8. Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, et al. Very long-term results (more than 20 years) of valve repair with Carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001; 104(12 Suppl 1):I8–I11. PMID: 11568021.

9. Choudhary SK, Talwar S, Dubey B, Chopra A, Saxena A, Kumar AS. Mitral valve repair in a predominantly rheumatic population. Longterm results. Tex Heart Inst J. 2001; 28:8–15. PMID: 11330754.
10. Suri RM, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, Mullany CJ, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg. 2006; 82:819–826. PMID: 16928491.

11. Chauvaud S, Fuzellier JF, Berrebi A, Deloche A, Fabiani JN, Carpentier A. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation. 2001; 104(12 Suppl 1):I12–I15. PMID: 11568022.

12. Duran CM, Gometza B, Saad E. Valve repair in rheumatic mitral disease: an unsolved problem. J Card Surg. 1994; 9(2 Suppl):282–285. PMID: 8186582.

Fig. 1
Preoperative two-dimensional (2D) transthoracic echocardiography. Two-chamber views with 2D and color Doppler showing severe mitral regurgitation due to a coaptation defect between the anterior and posterior mitral leaflets. The regurgitant flow arising from the tip of the AML in these views (arrow) suggests a defect in the free margin of the AML tip. LA: left atrium, LV: left ventricle, AML: anterior mitral leaflet (Video images are available on-line, video link 1 and 2).
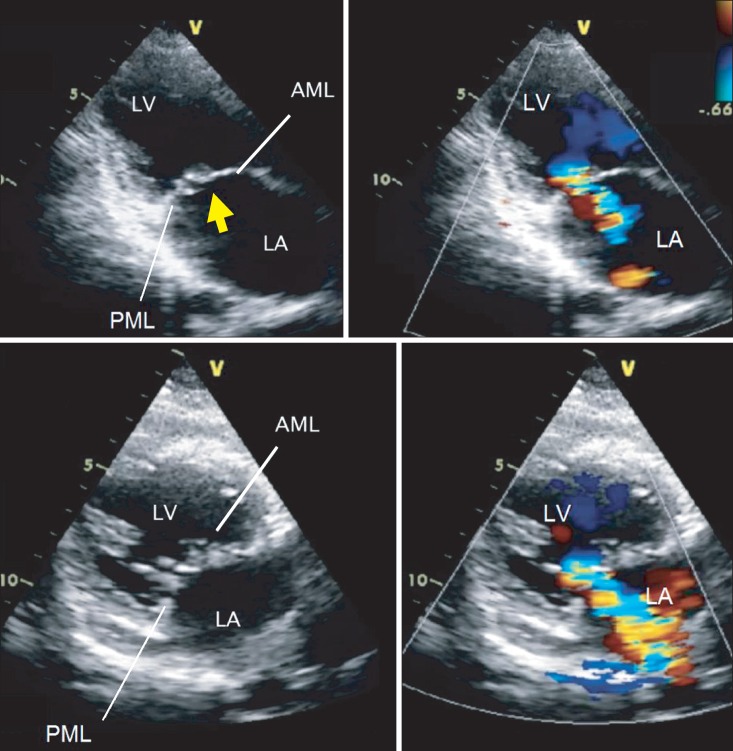
Fig. 2
Intraoperative two-dimensional transesophageal echocardiography. Midesophageal (ME) long-axis (LAX) and commissural views with color Doppler showing severe regurgitation between the mid-part of anterior and posterior mitral leaflets (A2 and P2 scallops). The free margin of the AML tip appears intact in the ME LAX view. Central regurgitant flow arising from the mid scallop gap, A2 or P2, is noted in the ME commissural view. However, it was difficult to make a definite diagnosis regarding which part was responsible for the concurrent coaptation defect of the mitral valve producing mitral regurgitation using these views. LA: left atrium, LV: left ventricle, Ao: aorta, AML: anterior mitral leaflet, PML: posterior mitral leaflet. P1: anteriolateral scallop of PML, P2: middle scallop of PML, P3: posteromedial scallop of PML, and A2: middle part of AML (Video images are available on-line, video link 3 and 4).
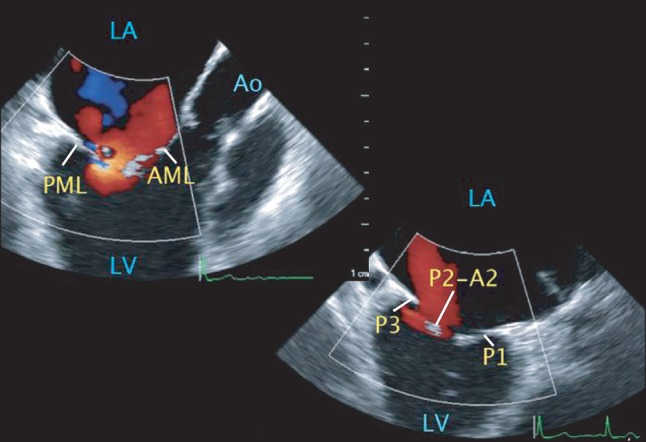
Fig. 3
Intraoperative pre-procedural real-time three-dimensional transesophageal echocardiography. Pre-procedural "en face view" of the mitral valve in the left atrial perspective, depicting the "surgeon's view," showing a large defect in the mid-part of the posterior mitral leaflet (P2 scallop), fusion of both anterolateral and posteromedial commissures, and a ruptured chorda of the P3 scallop. AML: anterior mitral leaflet, ALC: anterolateral commissure, PMC: posteromedial commissure. P1: anterolateral scallop of posterior mitral leaflet, P3: posteriomedial scallop of posterior mitral leaflet (Video images are available on-line, video link 5).
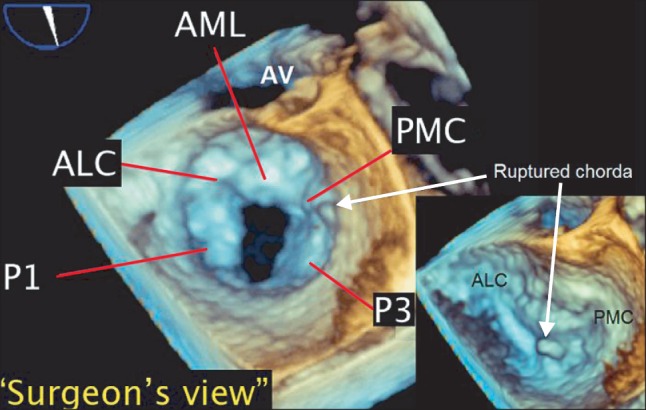
Fig. 4
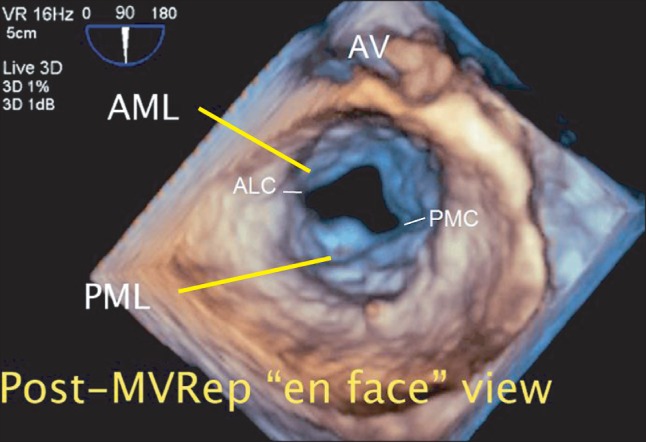
Intraoperative post-procedural real-time three-dimensional transesophageal echocardiography. The "en face" view of the mitral valve in the left atrial perspective immediately after the mitral valve repair procedure (MVRep) showing the successfully repaired posterior mitral leaflet and enlarged mitral valve opening. AML: anterior mitral leaflet, PML: posterior mitral leaflet, ALC: anterolateral commissure, PMC: posteromedial commissure (Video images are available on-line, video link 6).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download