Abstract
Cortriatriatum is a rare congenital cardiac disorder with fibromuscular band (diaphragm) dividing the left atrium (LA) into the proximal and distal parts. Surgical correction of cortriatriatum requires full preoperative evaluation of the structural anomalies including the LA diaphragm and their pathophysiology. In the present case, a 44 year-old lady diagnosed as cortriatriatum underwent surgical correction. Intraoperative three-dimensional transesophageal echocardiography provided detailed information regarding the shape and extent of the LA diaphragm, which had been partially evaluated by preoperative two-dimensional transthoracic and transesophageal echocardiography, and facilitated the intraoperative patient management and surgical decision making.
Cortriatriatum is a very rare congenital heart disease (about 0.1%) with fibromuscular band (diaphragm) in the left atrium (LA), which forms an abnormal septum dividing LA into proximal and distal chambers: the proximal chamber is connected to pulmonary venous network whereas the distal chamber consists of LA appendages and mitral valve. The LA diaphragm usually has one or more holes (diaphragmatic defects) connecting the proximal and distal LA chambers. However, in cases of complete septation, additional congenital anomalies including atrial septal defect are required for enabling pulmonary venous blood to drain into the distal LA chamber. Since the clinical features and strategy for surgical correction of cortriatriatum are closely related to the characteristics of the LA diaphragm and resultant intracardiac blood flow pattern, a thorough understanding of the exact shape of the abnormal LA diaphragm related to associated cardiac anomalies is mandatory in managing cortriatriatum [1,2].
Meanwhile, although preoperative diagnosis of cortriatriatum is usually made with preoperative two-dimensional (2D) echocardiography, the complexity of the LA diaphragm with polymorphic attachments to the LA wall as well as the difficulties in aligning 2D images of echocardiography requires additional diagnostic imaging modalities for full preoperative evaluation of cortriatriatum. In the present case, intraoperative application real time (RT) three-dimensional (3D) transesophageal echocardiography (TEE) provided additional information regarding the shape and extent of the LA diaphragm and facilitated easier and faster evaluation of pathophysiology and surgical decision during a surgical correction procedure for cortriatriatum.
A 44-year-old female underwent elective tricuspid valve repair and atrial septal defect (ASD) closure due to known congenital cardiac anomalies suggesting cortriatriatum. These anomalies comprised tricuspid insufficiency, interatrial septal defects, and an abnormal membrane in the LA in preoperative 2D TEE. She presented with peripheral edema, especially in the lower extremities, frequent upper respiratory tract infections and mild exertional dyspnea without any other remarkable medical history.
Anesthetic induction and tracheal intubation was performed using target-controlled infusion (TCI) of propofol (effect site 2.0 mcg/ml) and remifentanil (plasma 15-20 ng/ml) as well as bolus rocuronium 0.9 mg/kg, adjustment of TCI-propofol (effect site concentration of 1.0-1.2 mcg/ml to maintain BIS value 40-60) and cisatracurium infusion (0.2 mcg/kg/min) were followed for anesthesia maintenance. After anesthetic induction, pulmonary artery catheter placement was placed in the right internal jugular vein under ultrasound-guidance. A 3D TEE probe (X7-2t transducer; Philips Healthcare, Andover, MA, USA) was placed and connected to TEE console (iE33™, Philips Healthcare, Andover, MA, USA), intraoperative 2D and 3D TEE examinations were then performed. The 2D TEE examination showed an enlarged right atrium (RA); a relatively small and septated LA with a solid, thin echogenic membrane (indicating the LA diaphragm) separating the LA into two distinct chambers; a larger (proximal) LA chamber in the superior aspect; and a small (distal) LA chamber in the inferior aspect (Fig. 1). The distal LA chamber had a mitral valve (MV) apparatus and communicated with the left ventricle (LV). Although intraoperative 2D TEE findings corresponded quite well with preoperative TTE findings, they could not provide the detailed information regarding the extent of the LA diaphragm and the shape of the diaphragmatic defect. Important information for understanding the intracardiac flow pattern of the systemic and pulmonary circulation could not be provided by using 2D TEE alone. Additional real time 3D "en face" image and cropped images from the real time 3D volume images facilitated a complete understanding of the whole features of the complex anomalies of this case, including the shape and size of the diaphragm in a considerably easier and faster manner. 3D TEE showed that the RA dimension was much larger than the LA dimension with a thin membranous diaphragm traversing the whole LA to form a horizontal roof between the proximal and distal LA chambers. The LA diaphragm did not have any holes: the proximal LA chamber communicated with the RA through a 2 cm defect in the interatrial septal area, which allowed the drainage of pulmonary venous blood from the proximal LA into the enlarged RA and thus contributed to the role of the RA as a mixing chamber for systemic, pulmonary, and coronary venous blood. The mixed blood in the RA then flowed through the tricuspid valve to reach the right ventricle, as well as through a 4 cm ASD to reach the distal LA chamber. The relatively small distal LA served as a functional LA with a left atrial appendage and MV apparatus at the bottom (Fig. 2).
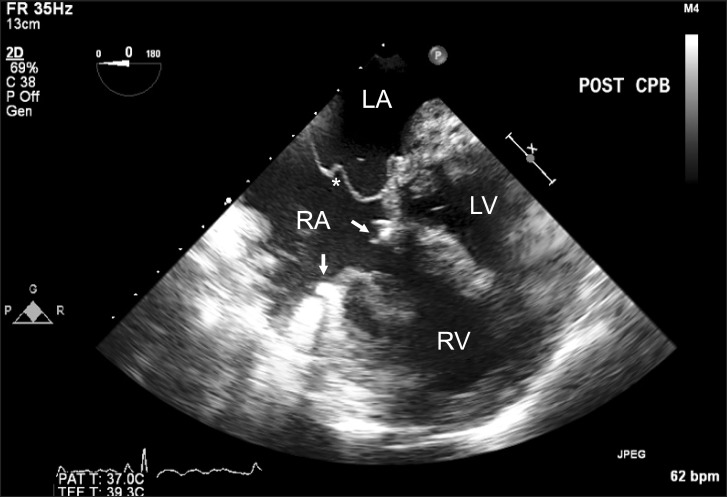
Right atriotomy was performed through a median sternotomy after initiation of moderate hypothermic cardiopulmonary bypass. A self-retaining retractor was used to maximize the exposure of all intracardiac structures, including the LA diaphragm, atrioventricular valve apparatus, and coronary and pulmonary venous openings. After confirming the above determinations by surgical perspectives, the following procedures were performed; full resection of the LA diaphragm, tricuspid valve ring annuloplasty, removal of the diaphragmatic defect by constructing of a new interatrial septum using a bovine pericardial patch, and closure of the ASD (Fig. 3). The newly constructed septum separated the pulmonary and systemic flow which draining into LA and RA, respectively.
In cortriatriatum, the restricted flow to the functional LA (distal LA chamber) due to the abnormal LA diaphragm produces various symptoms similar to those seen in MV stenosis and heart failure. Cyanosis can be developed in the presence of large septal defect with the right to left shunt.
Differential diagnosis of cortriatriatum includes total anomalous pulmonary venous connection, congenital stenosis of pulmonary veins, atresia of common pulmonary veins, supravalvular stenosis of the left atrium and congenital mitral stenosis. In adults, it should also be differentiated from rheumatic mitral stenosis, left atrium tumor or thrombus or persistence of left superior vena cava which is draining into coronary sinus. Cortriatriatum with pulmonary venous drainage into the proximal LA chamber requires differential diagnosis from total anomalous pulmonary venous connection (TAPVC) with pulmonary venous drainage into the coronary sinus or RA. TAPVC requires an interatrial communication for survival, whereas cortriatriatum uses various routes such as holes in the diaphragm or a patent fossa ovalis to connect the proximal LA to the distal (functional) LA chamber. Cortriatriatum also must be differentiated from a supravalvular mitral ring, in which the left atrial appendage is above the LA diaphragm.
Among various imaging techniques, 2D echocardiography can be used to evaluate cortriatriatum without any risks related to radiation exposure. However, it requires capturing multiple well-aligned 2D images for creation of appropriate imaginary or virtual 3D cardiac structures, and tremendous effort may be required to align the image planes. Furthermore, the production of appropriate 2D images is impossible in certain cases of complex cardiac anomalies.
Full evaluation of the shape and extent of the LA diaphragm as well as diaphragmatic defect may be difficult by using 2D echocardiography alone, as shown in the present case. The lack of comprehensive 2D images containing sufficient information in the present case was probably due to the complexity of the LA diaphragm, with polymorphic attachments to the LA wall, and the limitation of aligning 2D images, especially by using 2D TEE. The real-time 3D echocardiography is especially beneficial in evaluating cardiac structures located near to TEE probe with favorable alignment (perpendicular to sonographic beam), such as MV and LA [3]. Even a single "en face" image from real-time 3D TEE would be able to present a true anatomic perspective of objective cardiac structures which had been shown in this case, and understanding of the detailed structures much easier and faster [2,4,5]. Preoperative 3D TTE as well as other imaging modalities, such as Magnetic Resonance Imaging or Computed Tomography scan, might be useful in evaluating full-spectrum of cortriatriatum as a provider of additional 3D information.
Anesthetic management for cortriatriatum is not much different from that for TAPVC or mitral stenosis, however, caution should be placed for avoiding possible entrapment of central venous catheter into abnormal chamber or connecting orifices. Postoperative course usually uncomplicated even though some patients may have low cardiac output state in case of association with other congenital anomalies.
In conclusion, the present case demonstrates the clinical efficacy of intraoperative RT 3D TEE in enabling full evaluation of the structural anomalies and pathophysiology of cortriatriatum by providing additional and precise information in a much easier and faster manner.
Acknowledgments
This paper was supported by Konkuk University School of Medicine and Konkuk University Medical Center.
References
1. Modi KA, Annamali S, Ernest K, Pratep CR. Diagnosis and surgical correction of cor triatriatum in an adult: combined use of transesophageal and contrast echocardiography, and a review of literature. Echocardiography. 2006; 23:506–509. PMID: 16839391.

2. Hoffmann R, Lambertz H, Flachskampf FA, Hanrath P. Transoesophageal echocardiography in the diagnosis of cor triatriatum; incremental value of colour Doppler. Eur Heart J. 1992; 13:18–20. PMID: 1327795.
3. Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, Bednarz JE, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr. 2006; 19:413–421. PMID: 16581480.

4. Hanna R, Chen MA, Gill EA. Cor triatriatum evaluated by real time 3D TEE. Echocardiography. 2011; 28:E125–E128. PMID: 21349109.

5. Willens HJ, Ferrer PL, Tamer DF, Labrador E, Aqatston AS, Keith K, et al. Cor triatriatum sinister in an adult: management guided by real time three-dimensional transesophageal echocardiography and stress echocardiography. Echocardiography. 2010; 27:E132–E136. PMID: 20553320.

Fig. 1
Preprocedural two-dimensional transesophageal echocardiographic images. Mid-esophageal four-chamber view (left) and its orthogonal image (right) show an abnormal membrane (diaphragm, indicated by *) horizontally traversing the LA and dividing it into two distinct chambers: a large superior (proximal LA) and a small inferior (distal LA) chamber. RA: right atrium, RV: right ventricle, LA: left atrium, LV: left ventricle, CS: coronary sinus. *LA membrane.
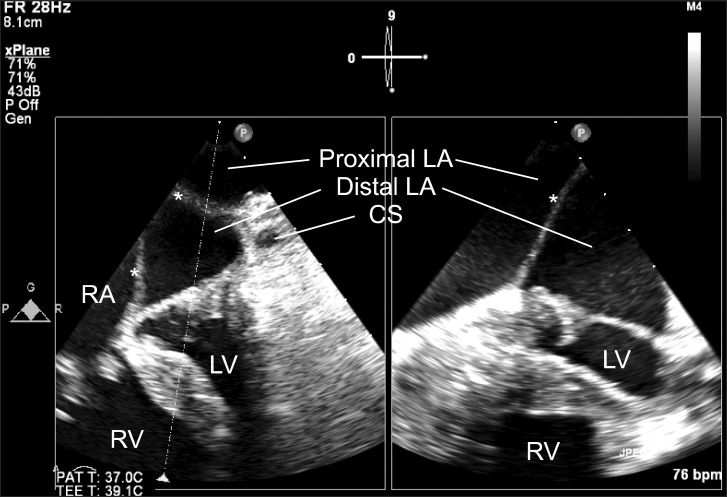
Fig. 2
Preprocedural three-dimensional transesophageal echocardiography. The "en face" views of both atria delineate well the whole shape of the left atrial diaphragm (white arrows) and facilitate quicker and easier understanding of its relationship with adjacent structures. The images arranged as their order of sequential cropping of the 3D en face view (a higher level en face image is in the left upper image, a lower level is in the right lower image). The enlarged right atrium (RA) acts as a mixing chamber of pulmonary and systemic venous returns. The diaphragmatic defect (*) at the medial end of the diaphragm connects the proximal left atrial chamber (proximal LA) and RA; the 4-cm atrial septal defect (ASD) connects the RA and the distal left atrial chamber (distal LA). The distal LA has a left atrial appendage and acts as a functional LA, connecting to the left ventricle through the mitral valve. RA: right atrium, ASD: atrial septal defect, LAA: left atrial appendage, LA: left atrium, MV: mitral valve, LV: left ventricle.
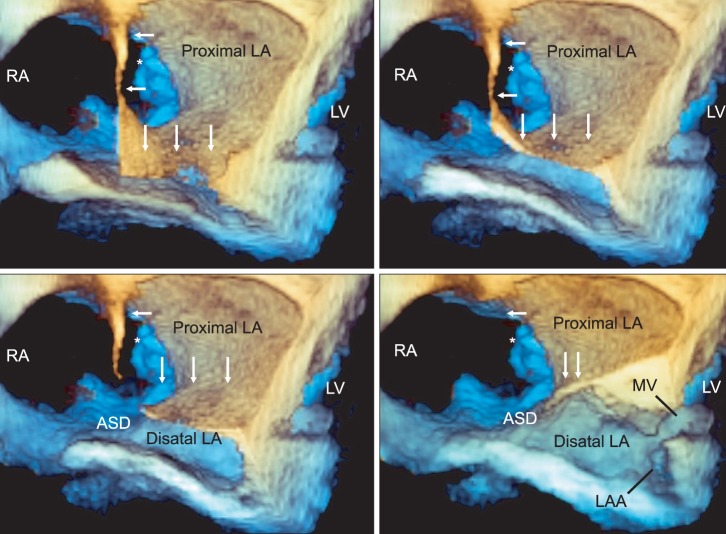
Fig. 3
Postprocedural two-dimensional transesophageal echocardiography. Mid-esophageal four-chamber view shows a newly constructed interatrial septum (*) separating the left and right circulation and a tricuspid valve repair ring (arrows). RA: right atrium, RV: right atrium, LA: left atrium, LV: left ventricle.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download