Abstract
Background
Lidocaine is a useful intravenous and topical adjunct to facilitate tracheal intubation. We evaluated the effect of tracheal lidocaine on tracheal intubating conditions without neuromuscular blocking agent and hemodynamics during anesthesia induction with propofol and remifentanil target-controlled infusion (TCI).
Methods
Fifty patients, aged 18-60 years, scheduled for closed reduction of fractured nasal bone were randomly assigned to the control group (n = 25) or lidocaine group (n = 25). Anesthesia was induced with propofol-remifentanil TCI with the effect-site concentration of 5 µg/ml and 5 ng/ml. Four minutes after the start of propofol-remifentanil TCI, 4% lidocaine or saline 3 ml was instilled to larynx and trachea, and intubation was performed 1 min later. Acceptable intubation was defined as excellent or good intubating conditions. Hemodynamic data, induction and recovery profiles were recorded.
Results
Intubating condition was clinically acceptable in 13 out of 25 (52%) patients in the control group and in 22 out of 25 (88%) in the lidocaine group, and there was a significant difference between the two groups in regard to acceptable intubating conditions (P = 0.005). Mean arterial pressure change over time was significantly different between the two groups. There were no significant differences in the heart rate between the two groups.
A target-controlled infusion (TCI) of propofol has been used for day-case anesthesia, and it provides a smooth induction, good control of intraoperative condition, a rapid recovery and a low incidence of post-operative nausea and vomiting (PONV). In addition, co-administration of propofol and remifentanil has been shown to provide conditions for successful tracheal intubation without the use of a neuromuscular blocking drug [1-3]. Tracheal intubation without neuromuscular blocking drugs may be used in cases where tracheal intubation is necessary but prolonged muscle relaxation is not, such as in short ambulatory surgery. However, avoidance of neuromuscular blocking drugs may increase the risk of difficult tracheal intubation [4], and trauma to the airway can occur if laryngoscopy and intubation are attempted under unsuitable conditions (e.g., poor jaw relaxation, closed vocal cords).
When using propofol and remifentanil TCI without neuromuscular blockade for tracheal intubation, the main reason for rating failed intubation is coughing rather than unfavorable vocal cord or laryngoscopic scores [3]. This suggests that the primary stressor during tracheal intubation is the trachea stimulus [5,6]. However, relatively higher anesthetic concentrations to obtund airway reflexes for tracheal intubation inevitably produce adverse hemodynamic events [7,8]. Laryngotracheal lidocaine could attenuate cardiovascular responses to endotracheal intubation [9], and improve intubating conditions without muscle relaxants [10]. In addition, total intravenous anesthesia (TIVA), in contrast to volatile anesthesia, has the advantage of maintaining steady anesthetic depth during the application of laryngotracheal lidocaine. In this randomized, double-blind study, we evaluated the effect of laryngotracheal lidocaine on the intubating conditions and hemodynamic responses for tracheal intubation during propofol and remifentanil TCI without neuromuscular blocking agent in day-case anesthesia.
This study was approved by the Institutional Review Board, and registered at ClinicalTrials.gov. Written informed consent for the study was obtained from all patients. We enrolled American Society of Anesthesiologists physical status I or II, aged 18-60 years, undergoing day-case surgery for nasal bone fracture. Patients with a history of reactive airway disease, predicted difficult intubation (Mallampati class III-IV, history of difficult intubation), and obesity (body mass index > 30 kg/m2) were excluded from the study. No premedication was administered prior to surgery. Upon arrival in the operating room, all patients were monitored with electrocardiogram, pulse oximeter, and noninvasive blood pressure and bispectral index (BIS) (BIS VISTA™ monitor, four electrode sensor; Aspect Medical Systems, Norwood, MA, USA). Using a computer generated randomization table, 50 patients were randomly assigned to the lidocaine group (n = 25) or control group (n = 25). Following injection of iv 0.2 mg glycopyrrolate, anesthesia was induced with propofol TCI at an effect-site concentration of 5.0 µg/ml and remifentanil TCI at an effect-site concentration of 5.0 ng/ml, respectively, using a two-channel TCI pump (Orchestra®, Fresenius Vial, Brezins, France). The pharmacokinetic sets used for calculation of target effect-site concentrations for propofol and remifentanil were Marsh et al. [11] and Minto et al. [12] models, respectively. Four minutes after induction, 4% lidocaine or normal saline 3 ml was applied with a spray tip attached to 5 ml syringe into the larynx (1 ml) and trachea (2 ml) under direct vision with a standard Macintosh laryngoscope. Tracheal intubation was performed 1 min after lidocaine instillation by an experienced anesthesiologist. Endotracheal tubes with an internal diameter of 7.0 mm were used for female patients and tubes with an internal diameter of 8.0 mm were used for male patients. Intubating conditions were evaluated according to a scoring system described by Fuchs-Buder et al. [13]: ease of laryngoscopy (Easy, Fair, Difficult), vocal cord position (Abducted, Intermediate/moving, Closed) and reaction to insertion of the tracheal tube and cuff inflation (diaphragmatic movement-coughing) (None, Slight; 1 to 2 weak contractions or movement for less than 5 s, Vigorous; more than 2 contractions and/or movement for longer than 5 s). Each of these variables was rated as excellent, good, or poor. Intubating conditions were excellent if all variables were excellent; they were good if at least one variable was good and the rest were excellent, and they were poor if any variable was poor. Acceptable intubation was defined as excellent or good intubating conditions. The anesthesiologist who performed the intubations and who assessed the intubating conditions was blinded regarding group assignment. If intubation failed, target concentration of propofol and remifentanil increased to 6 to 8 µg/ml and 6 to 8 ng/ml, respectively, and tracheal intubation was again attempted. Clinically significant hypotension and bradycardia during anesthesia induction were defined as a mean arterial pressure (MAP) of < 55 mmHg and a heart rate (HR) of < 45 beats/min, respectively. These conditions were treated with atropine or ephedrine where appropriate. At the end of surgery, propofol and remifentanil infusion stopped, and manual ventilation was begun with 100% oxygen. Extubation was performed in a standard manner when patients were able to open their eyes, squeeze a hand, and lift their head on command. Incidence of PONV and visual analogue scale (VAS; 0 for no pain, and 10 for the worst possible pain) were measured at 15 min after arrival in the post-anesthesia care unit (PACU). Postoperative pain was treated with iv 30 mg ketorolac, if the VAS score was > 6, or patients wanted analgesics. Patients were discharged from PACU after modified Aldrete score [14] of ≥ 9 was noted. Hemodynamic data and BIS were recorded before induction, 2 min and 4 min after induction, and 2 min after intubation. Time to loss of consciousness (the interval between the start of TCI and loss of responsiveness to verbal command to open eyes every 10 s), operation time, anesthesia time, eye opening time (time from end of operation to eye opening), extubation time (time from end of operation to extubation), and coughing count at extubation were also measured.
Based on a previous study [7], we expected that the rate of clinically acceptable intubating conditions in the control group would be 35% which would improve to 80% with topical lidocaine. We needed 23 patients in each group to achieve 80% power and 5% significance level. To allow for dropouts, we increased the sample size to 25 patients per group. Statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± SD or number of patients. Patient characteristics and induction, recovery profiles were compared using Student's t-test. Categorical data were analyzed using chi-square test. Changes in hemodynamic data over time between the groups were compared by repeated measures ANOVA. A P value < 0.05 was considered significant.
A total of 56 patients were screened for eligibility, and 6 patients were excluded, leaving 50 patients to be randomized and complete the study. There were no significant differences in the patients' characteristics between the two groups (Table 1). There were no significant differences in the induction and recovery profiles between the two groups in the operating room and PACU (Table 2).
The overall intubating condition was regarded as clinically acceptable (excellent or good) in 13 out of 25 (52%) patients in the control group and in 22 out of 25 (88%) in lidocaine group, and there was a significant difference between two groups for acceptable intubating condition (P = 0.005) (Table 3). Intubating conditions were excellent in 6/25 patients in the control group and 14/25 patients in the lidocaine group (P = 0.021). Intubating conditions were poor in 12/25 and 3/25 patients in the control and lidocaine groups, respectively (P = 0.005). In the control group, the most common cause of poor intubating conditions was vigorous coughing.
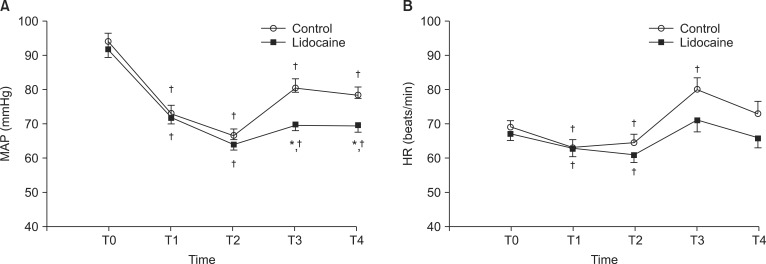
Fig. 1 illustrates the changes in MAP and HR during induction of anesthesia. MAP change over time was significantly different between the two groups (P = 0.033). MAP was significantly higher in the control group than in the lidocaine group immediately and 2 min after intubation. Compared with the baseline values (T0), MAP decreased significantly from 2 min after induction (T1) to 2 min after intubation (T4) in both groups. HR decreased significantly from 2 min after induction to 4 min after induction compared to the baseline value in both groups, and increased at immediately after intubation in the control group. There were no adverse respiratory events, such as laryngospasm, and SpO2 remained above 96% in all patients. One patient in each group was given ephedrine 4 mg due to clinically significant hypotension. No significant difference was observed in BIS between the two groups during the study period (data not shown). At 4 min after anesthesia induction, mean ± SD of BIS was 53 ± 13 and 51 ± 9 in the control and lidocaine groups, respectively, and there was no significant difference (P = 0.49).
This study demonstrated that laryngotracheal administration of 4% lidocaine could achieve better intubation conditions in day-case anesthesia using propofol and remifentanil without a neuromuscular blocking agent, and attenuate the pressor response to tracheal intubation.
Propofol and remifentanil have several properties, which make them potentially useful as day-case anesthetics. The use of TIVA with propofol and remifentanil resulted in significantly fewer episodes of PONV compared with sevoflurane, and was associated with earlier awakening in day-case anesthesia [15]. However, because high concentration of remifentanil or propofol TCI to obtund airway reflexes for tracheal intubation is related to frequent hypotension and bradycardia requiring treatment [16] or delayed awakening, laryngotracheal lidocaine spray was chosen for cough suppression in this study. Previous study by Bülow et al. [10] showed that, when compared with saline spray, laryngotracheal lidocaine spray of 160 mg provided satisfactory intubating conditions from 73 to 100% during anesthesia induction using propofol 2.5 mg/kg and alfentanil 30 µg/kg without muscle relaxants. Our study showed that the administration of lidocaine 120 mg into larynx and trachea during propofol-remifentanil TCI improved acceptable intubating conditions from 52 to 88% in adult patients without hemodynamic perturbations. In addition, the proportion of excellent intubating conditions was greater in the lidocaine group than in the control group. The major cause of unacceptable intubation condition was coughing in the control group. This was consistent with earlier studies evaluating intubating conditions without neuromuscular blockade during propofol-remifentanil TCI [3,16].
In this study, it is likely that cough suppression by tracheal lidocaine resulted from local effects. Hamaya and Dohi [17] suggested that the inhibition of airway tactile stimulation with topical lidocaine could be mainly due to direct blockade of the mechanoreceptors of the airways and partly to its systemic effect. In addition, they found that the peak serum lidocaine concentration is at 5 to 10 min after laryngeal application of 200 mg lidocaine [17]. Since we used a lidocaine dose of 120 mg and the interval 60 s is shorter than that to peak concentration, the systemic effect of lidocaine is either small or negligible. Therefore, the probable reason for improvement in the intubation conditions seems to be the local, not the systemic, anesthetic effect of lidocaine. Intravenous lidocaine has been reported to be a useful adjunct to suppress the cough reflex during tracheal intubation without neuromuscular blockade. Yukioka et al. [18] note that intravenous administration of lidocaine 2 mg/kg at 1 min before intubation completely suppressed cough reflex. However, though no patient with lidocaine side effects was noted in their study, the authors conclude that this dose may produce systemic toxicity, because some patients showed high blood concentration of 8 µg/kg.
Several studies have examined the efficacy of tracheal lidocaine in attenuating the hemodynamic responses to endotracheal intubation [9,19,20], with inconsistent results, depending on the timing of lidocaine administration. Tracheal administration of lidocaine 1 min before tracheal intubation was ineffective for attenuation of the cardiovascular response to intubation [19,20]. However, tracheal lidocaine attenuated pressor responses to intubation when the tracheal intubation was performed more than 2 min after a tracheal spray in a study by Takita et al. [9]. They suggested that sufficient time is needed for tracheal lidocaine to attenuate the hemodynamic responses to laryngoscopy and intubation. In our study, tracheal lidocaine spray 1 min before tracheal intubation was performed could reduce, but not abolish the pressor response to tracheal intubation during propofol-remifentanil TCI.
There are some limitations in this study. First, percentage of acceptable intubating conditions in the control group was low, only 52%, probably due to shallow depth of anesthesia. However, although the higher effect-site concentration of remifentanil could increase the acceptable intubation condition rate of the control group, its higher doses would have been implicated in hemodynamic instability and chest wall rigidity before tracheal intubation during propofol induction. Previous studies reported significant decreases in MAP and HR before intubation during propofol induction in combination with the higher doses of remifentanil without neuromuscular blockade [7,21]. Second, we did not confirm that the improvement in intubating conditions by laryngotracheal lidocaine spray would reduce postoperative laryngeal morbidity, because postoperative laryngeal sequelae were not evaluated in this study. However, better intubating condition has been reported to be associated with lower incidence of postoperative hoarseness and vocal cord injuries [22]. Further studies exploring the association between lidocaine spray and laryngeal morbidity might be needed.
In conclusion, in day-case anesthesia, laryngotracheal administration of lidocaine is an effective method for neuromuscular blocking agent-free tracheal intubation during propofol remifentanil TCI without increasing the risk of hypotension during anesthesia induction.
References
1. Alexander R, Olufolabi AJ, Booth J, El-Moalem HE, Glass PS. Dosing study of remifentanil and propofol for tracheal intubation without the use of muscle relaxants. Anaesthesia. 1999; 54:1037–1040. PMID: 10540091.

2. Woods AW, Grant S, Harten J, Noble JS, Davidson JA. Tracheal intubating conditions after induction with propofol, remifentanil and lignocaine. Eur J Anaesthesiol. 1998; 15:714–718. PMID: 9884858.

3. Ithnin F, Lim Y, Shah M, Shen L, Sia AT. Tracheal intubating conditions using propofol and remifentanil target-controlled infusion: a comparison of remifentanil EC50 for Glidescope and Macintosh. Eur J Anaesthesiol. 2009; 26:223–228. PMID: 19237984.

4. Lundstrøm LH, Møller AM, Rosenstock C, Astrup G, Gätke MR, Wetterslev J. Avoidance of neuromuscular blocking agents may increase the risk of difficult tracheal intubation: a cohort study of 103,812 consecutive adult patients recorded in the Danish Anesthesia Database. Br J Anaesth. 2009; 103:283–290. PMID: 19457894.
5. Takahashi S, Mizutani T, Miyabe M, Toyooka H. Hemodynamic responses to tracheal intubation with laryngoscope versus lightwand intubating device (Trachlight) in adults with normal airway. Anesth Analg. 2002; 95:480–484. PMID: 12145076.

6. Hirabayashi Y, Hiruta M, Kawakami T, Inoue S, Fukuda H, Saitoh K, et al. Effects of lightwand (Trachlight) compared with direct laryngoscopy on circulatory responses to tracheal intubation. Br J Anaesth. 1998; 81:253–255. PMID: 9813535.

7. Stevens JB, Wheatley L. Tracheal intubation in ambulatory surgery patients: using remifentanil and propofol without muscle relaxants. Anesth Analg. 1998; 86:45–49. PMID: 9428849.
8. Troy AM, Huthinson RC, Easy WR, Kenney GN. Tracheal intubating conditions using propofol and remifentanil target-controlled infusions. Anaesthesia. 2002; 57:1204–1207. PMID: 12479190.

9. Takita K, Morimoto Y, Kemmotsu O. Tracheal lidocaine attenuates the cardiovascular response to endotracheal intubation. Can J Anaesth. 2001; 48:732–736. PMID: 11546711.

10. Bülow K, Nielsen TG, Lund J. The effect of topical lignocaine on intubating conditions after propofol-alfentanil induction. Acta Anaesthesiol Scand. 1996; 40:752–756. PMID: 8836274.
11. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991; 67:41–48. PMID: 1859758.

12. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86:10–23. PMID: 9009935.
13. Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007; 51:789–808. PMID: 17635389.

14. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995; 7:89–91. PMID: 7772368.

15. Hong JY, Kang YS, Kil HK. Anaesthesia for day case excisional breast biopsy: propofol-remifentanil compared with sevoflurane-nitrous oxide. Eur J Anaesthesiol. 2008; 25:460–467. PMID: 18298873.
16. Kim SJ, Yoo KY, Park BY, Kim WM, Jeong CW. Comparison of intubating conditions and hemodynamic responses to tracheal intubation with different effect-site concentrations of remifentanil without muscle relaxants during target-controlled infusion of propofol. Korean J Anesthesiol. 2009; 57:13–19.

17. Hamaya Y, Dohi S. Differences in cardiovascular response to airway stimulation at different sites and blockade of the responses by lidocaine. Anesthesiology. 2000; 93:95–103. PMID: 10861151.

18. Yukioka H, Yoshimoto N, Nishimura K, Fujimori M. Intravenous lidocaine as a suppressant of coughing during tracheal intubation. Anesth Analg. 1985; 64:1189–1192. PMID: 4061901.

19. Mostafa SM, Murthy BV, Barrett PJ, McHugh P. Comparison of the effects of topical lignocaine spray applied before or after induction of anaesthesia on the pressor response to direct laryngoscopy and intubation. Eur J Anaesthesiol. 1999; 16:7–10. PMID: 10084094.

20. Derbyshire DR, Smith G, Achola KJ. Effect of topical lignocaine on the sympathodrenal responses to tracheal intubation. Br J Anaesth. 1987; 59:300–304. PMID: 3828178.

21. Klemola UM, Mennander S, Saarnivaara L. Tracheal intubation without the use of muscle relaxants: remifentanil or alfentanil in combination with propofol. Acta Anaesthesiol Scand. 2000; 44:465–469. PMID: 10757583.

22. Mencke T, Echternach M, Kleinschmidt S, Lux P, Barth V, Plinkert PK, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology. 2003; 98:1049–1056. PMID: 12717124.
Fig. 1
(A) The changes in mean arterial pressure (MAP) and (B) heart rate (HR) during anesthesia induction. Error bar means standard error. T0: before anesthesia induction, T1: 2 min after induction, T2: 4 min after induction, T3: immediately after tracheal intubation, T4: 2 min after tracheal intubation. *P < 0.05 vs. control group. †P < 0.05 vs. T0 within the group.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download