Abstract
Carney complex is an autosomal dominant disorder that occurs due to a mutation in PRKAR1A, which encodes protein kinase A. The clinical features are multiple endocrine gland neoplasms, skin tumors, pigmented skin lesions, myxomas, and schwannomas. In Carney complex, the cardiac myxoma is a common co-morbidity. It occurs in multiples, during young age, regardless of gender and cardiac chamber and is known to recur frequently. Therefore there are high risks of adhesion and massive bleeding due to repeated surgeries. Such surgical risks account for over 50% of disease-specific mortality of Carney complex patients. Here, we present anesthetic experiences of myxoma removal surgery in two patients with Carney complex.
Carney complex is an autosomal dominant disorder that occurs due to a mutation in the gene subunit PRKAR1A, which encodes protein kinase A. Clinical features are multiple endocrine tumor, skin tumor, pigmented skin lesions, myxomas, and schwannomas [1,2]. Carney complex can cause multiple endocrine dysfunctions in the pituitary gland, thyroid gland, adrenal gland, and testicular gland and induce difficult airway from acromegaly [3,4] and hemodynamic instability from adrenal gland dysfunction [1,5,6].
Cardiac myxomas are also commonly accompanied [1,7,8]. There are increasing risks of cardiac blood flow obstruction, arrhythmia, embolization, and sudden death depending on the size and location of the myxoma [7-9]. Compared to general myxomas, this differs by having no link to sex or cardiac chambers, usually appearing during younger age and in multiple, with frequent relapses and a tendency to increase rapidly in size [7,8]. Thus most cases require immediate surgical treatment after diagnosis, which has led to high risks of adhesion and massive bleeding due to repeated surgeries, and such surgical risks account for over 50% of disease-specific mortality of Carney complex patients [1,2].
The authors present the anesthetic experiences of cardiac myxoma removal of two Carney complex patients with multiple endocrine tumors and multiple cardiac myxomas.
A 14-year old male had a past history of a total of three cardiac myxoma removal surgeries in the left atrium three years ago, in the right ventricle anterior papillary muscle and chordae two years ago, and in anterior mitral leaflet side of the left atrial wall, inferior atrial septum, and right ventricular outflow lateral aspect one year ago. He underwent a left orchiectomy for a sertoli cell tumor three years ago. He was diagnosed with Carney complex with the presence of adrenal mass, thyroid nodule, and pituitary adenoma, and was under ambulatory follow-up monitoring.
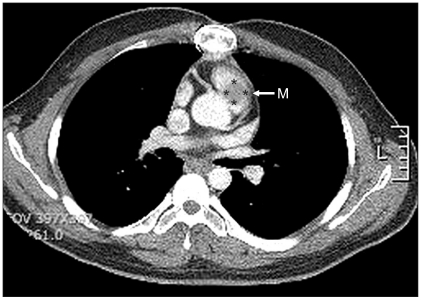
The pre-operative echocardiography revealed a 20 × 20 mm mobile mass in the right ventricular outflow tract (Fig. 1 and 2). It also showed a coronary sinus dilatation (18 × 13 mm), and the ejection fraction was 59.8%. Hemiparesis developed after the cardiac myxoma removal surgery three years ago, but the patient recovered without any complications. Using sellar magnetic resonance imaging, a 17 mm pituitary adenoma and old multifocal cerebral infarctions in the cerebellum, brainstem, both occipital lobes, temporal lobes, parietal lobes, thalami, corpus callusum, left frontal lobe, and left basal ganglia were seen. The electrocardiography showed a normal sinus rhythm with nonspecific intraventricular block, and chest radiography showed no active lung lesions.
For the pre-operative evaluation of the endocrine function, the department of endocrinology was consulted. A quadruple test was carried out by injecting growth hormone-releasing hormone (GHRH), corticotrophin-releasing hormone (CRH), thyrotropic releasing hormone (TRH), and gonodotropin releasing hormone (GnRH). The results were normal responses to growth hormone (GH), thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and adrenocorticotropic hormone (ACTH). However a decreased response was seen to cortisol. Adrenal insufficiency was suspected, so on the morning of surgery 250 mg of hydrocortisone was injected intravenously.
As premedication, 2 mg of midazolam and 0.2 mg of glycopyrrolate were intramuscularly injected and 20 mg of famotidine was intravenously injected. Physical examination revealed acromegaly due to the pituitary adenoma (height 191 cm, weight 104 kg) and the Mallampati class was 2. Preoperative vital signs showed blood pressure at 120/80 mmHg and heart rate at 72 bpm. A left radial arterial line was placed before anesthetic induction. Electrocardiogram, pulse oxymeter, and arterial blood pressure were monitored while 120 mg of propofol and 300 µg of fentanyl were slowly injected intravenously. After adequate mask ventilation was confirmed, 50 mg of rocuronium was intravenously injected. Although the patient presented with a large tongue and long mandibular length due to acromegaly, the Cormack-Lehane grade was II and endotracheal intubation was not difficult. Expecting severe adhesions from the multiple numbers of myxoma removal surgeries, a large bore intravenous line as well as the central line was maintained and the cell saver was prepared as provisions for massive bleeding. Transesophageal echocardiography and nasopharyngeal temperature monitor were set. Anesthesia was maintained with sevoflurane at 1-2 vol%, fentanyl 400 µg per hour, and rocuronium. During the operation, vital signs were stable with blood pressure at 100-120/50-60 mmHg and heart rate at 80-90 bpm.
A median sternotomy was performed at the incision site of the previous surgery. After detaching the tight pericardial adhesion, a cardiopulmonary bypass was performed. Three myxomatous nodules in the left atrium and one myxoma in the right atrium were removed. A 20 mm myxoma in the lateral wall of the right ventricular outflow tract was removed through the main pulmonary artery immediately below the pulmonary valve. The operation lasted 7 hours and 30 minutes and the cardiopulmonary bypass lasted 2 hours and 10 minutes.
After surgery, the patient was transferred to the intensive care unit where he was under observation for a day. The patient stabilized, so two days after he was transferred to the general ward. Post-operative echocardiography showed no remnant mass in the right ventricular outflow tract, but showed borderline concentric left ventricular hypertrophy with an ejection fraction of 69.2%.
A 16-year old male patient who was 172 cm in height and 73 kg in weight, and whose mother was diagnosed with Carney complex, had removal surgery of myxoma in the left atrium three years ago. He was diagnosed with Carney complex after presenting with a thyroid nodule, adrenal gland tumor, and sertoli cell tumor in the testicular gland. He also underwent left orchiectomy and left adrenalectomy two years ago. Afterwards, he had been under ambulatory follow-up monitoring for the thyroid nodule, pituitary adenoma, and Cushing's syndrome.
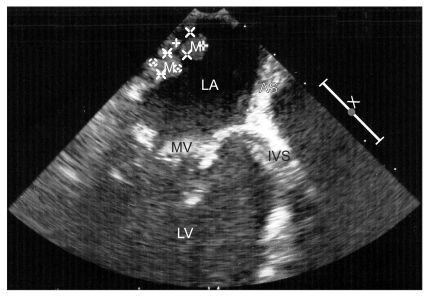
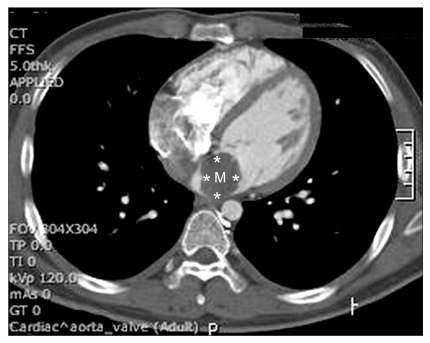
Pre-operative echocardiography confirmed a 34 × 24 mm sized myxoma in the posterior mitral valve leaflet of the left atrial posterior side, which is a rare location for myxomas to occur (Fig. 3 and 4). It also showed mild eccentric aortic regurgitation with an ejection fraction of 66.2%. Sellar magnetic resonance imaging showed a 1 cm sized pituitary adenoma on the fossa floor. Before the operation, the department of endocrinology and neurology were consulted. Because results of a quadruple test showed no abnormality in the pituitary functions, it was discussed that there would be no problem to continue on with myxoma removal surgery.
The electrocardiography showed sinus bradycardia with sinus arrhythmia, and the chest radiography showed no active lung lesions. Upon arrival on to the operating room, the blood pressure was 130/80 mmHg, and the heart rated was 78 bpm. The electrocardiography, pulse oxymeter, and arterial blood pressure were monitored as fentanyl 200 µg, etomidate 20 mg, and rocuronium 50 mg were administered for anesthetic induction. The patient was a Cormack-Lehane grade I, allowing easy endotracheal intubation. Intra-operative blood pressure was maintained at 140-160/60-80 mmHg and heart rate was 80 bpm. The surgery was performed as a median sternotomy, and with a cardiopulmonary bypass a 3 cm friable mass with a broad stalk in the posterior wall of the left atrium was removed through the right atrium. After surgery, the patient was transferred under sedation to the intensive care unit for observation. The patient stabilized, and two days later he was transferred to the general ward.
Both patients from the two cases described above were brothers with Carney complex. Their mother also had a history of numerous cardiac myxoma removal surgeries for Carney complex. At the molecular level, Carney complex is known to be related to genes located on q22-24 of chromosome 17 and p16 of chromosome 2. There is not much known about the 2p16, but over 70% of cases with typical phenotypes have PRKAR1A subunit mutation on 17q22-24. It encodes the protein kinase A (PKA) regulatory subunit and is the key component of the cAMP signaling pathway [1,7,8]. Patients with the PRKAR1A mutation compared to patients with no mutation have clinical patterns of myxomas, skin lesions, thyroid, and gonadal tumors at a younger age [1]. The two patients in present cases did not undergo chromosomal studies.
Because of the endocrine dysfunction from multiple endocrine tumors, there are many things to consider during preanesthetic evaluation and during anesthesia. For instance, growth hormone-producing pituitary adenoma occurs in 12% of the patients and is presented with acromegaly [1]. Acromegaly features include macroglossia, prognathism, enlargement and distortion (development of additional folds) of the glottis structure, hypertrophy of the soft tissues of the larynx and pharynx, and longer thyromental distance. The mask ventilation in patients with acromegaly compared to normal patients is known to be more difficult, and the rate of difficult intubation is known to be higher [3,4]. Therefore, preoperative airway evaluation is essential, such as the upper lip bite test, reported to be more accurate than the Mallampati classification predicting difficult intubation [4]. The patient in case 1 had findings of typical acromegaly, but endotracheal intubation was relatively easy.
Primary pigmented nodular adrenocortical disease is the most common form of adrenal gland tumor occurring in 60% of patients, and 25-30% of these patients show features of Cushing syndrome [1]. Cushing's syndrome due to primary pigmented adrenocortical disease appears to be adrenocorticotropic hormone-independent and its diagnosis is difficult because hypercortisolism progresses over several years [1,2]. Therefore, in diagnosing Carney complex, evaluation of the adrenal function and Cushing's syndrome related features such as fluidelectrolyte imbalance hypertension, diastolic dysfunction, glucose intolerance, and thromboembolic risk is essential [2,6].
Cardiac myxoma, a common co-morbidity, may cause cardiac blood flow obstruction (decreased filling of the left and right ventricle) [9]. Especially if the myxoma is located in the right ventricular outflow tract as in case 1, it may cause congestive heart failure due to pulmonary blood flow obstruction and right ventricle filling defect that may lead to peripheral edema, hepatomegaly, ascites, dyspnea, frequent syncope, and even sudden death [10,11]. Physical examination reveals jugular venous dilatation, ejection systolic murmur adjacent to left sternal border, diastolic murmur, 3rd heart sound, and delayed 2nd heart sound [10].
To prevent life-threatening complications, it is very important to prevent pulmonary embolization and pulmonary blood flow obstruction during anesthetic induction and maintenance. Drugs causing hemodynamic instability and inconvenient manipulations of the tumor and heart should be avoided. The occurrence of intra-operative central pulmonary artery embolism must be monitored with a transesophageal echocardiography [12]. In addition pre-anesthetic placement of the arterial line is imperative, and a large bore IV line and transfusion should be prepared as precautions for massive bleeding.
Because Carney complex is a multiple endocrine dysfunction comprising cardiac myxomas as well as other accompanying disorders, it requires thorough preparation by anesthesiologists. First, the pre-anesthetic evaluation of the presence of an accompanying disorder must be made. Second, an accurate evaluation of multiple endocrine function and management strategy are required. Thorough physical examination including that of the airway must be done and the location, size, clinical pattern of the myxoma must be checked. The cardiac function must also be evaluated. Third, the anesthesiologist must make careful preparations for events from difficult intubation to various hemodynamic changes due to adrenal gland tumor or cardiac myxoma at the time of anesthetic induction and maintenance. Finally, the anesthesiologist must be able to respond immediately and appropriately to sudden situations arising during anesthesia such as massive bleeding from repetitive surgeries.
References
1. Almeida MQ, Stratakis CA. Carney complex and other conditions associated with micronodular adrenal hyperplasias. Best Pract Res Clin Endocrinol Metab. 2010; 24:907–914. PMID: 21115159.

2. Rothenbuhler A, Stratakis CA. Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab. 2010; 24:389–399. PMID: 20833331.

3. Khan ZH, Rasouli MR. Intubation in patients with acromegaly: experience in more than 800 patients. Eur J Anaesthesiol. 2009; 26:354–355. PMID: 19401672.

4. Sharma D, Prabhakar H, Bithal PK, Ali Z, Singh GP, Rath GP, et al. Predicting difficult laryngoscopy in acromegaly: a comparison of upper lip bite test with modified Mallampati classification. J Neurosurg Anesthesiol. 2010; 22:138–143. PMID: 20118795.

5. Graham GW, Unger BP, Coursin DB. Perioperative management of selected endocrine disorders. Int Anesthesiol Clin. 2000; 38:31–67. PMID: 11100416.

6. Maciel RT, Fernandes FC, Pereira Ldos S. Anesthesia in a patient with multiple endocrine abnormalities. Case report. Rev Bras Anestesiol. 2008; 58:172–178. PMID: 19378536.
7. Bireta C, Popov AF, Schotola H, Trethowan B, Friedrich M, El-Mehsen M, et al. Carney-Complex: multiple resections of recurrent cardiac myxoma. J Cardiothorac Surg. 2011; 6:12. PMID: 21291531.

8. Teixeira R, Lourenco C, Coelho L, Vieira H, Ramos D, Castro G, et al. Carney complex: a case report. Rev Port Cardiol. 2009; 28:211–222. PMID: 19438156.
9. Liesting C, Ramjankhan FZ, van Herwerden LA, Kofflard MJ. Systemic embolisation as presentation and recurrence of cardiac myxoma two years after surgery. Neth Heart J. 2010; 18:499–502. PMID: 20978595.

10. Min PK, Park BE, Moon JY, Kang SM, Ha JW, Rim SJ, et al. Right Ventricular Myxoma Prolapsing into Pulmonary Artery with Significant Obstruction. J Korean Soc Echocardiogr. 2003; 11:42–45.

11. Paraskevaidis IA, Triantafilou K, Karatzas D, Kremastinos DT. Right ventricular multiple myxomas obstructing right ventricular outflow tract. J Thorac Cardiovasc Surg. 2003; 126:913–914. PMID: 14502195.

12. Tempe DK, Dutta D, Minhas H, Garg M, Virmani S. A rare case of myxoma in the right ventricular outflow tract extending to the pulmonary artery. Ann Card Anaesth. 2010; 13:167–168. PMID: 20442551.

Fig. 1
The pre-operative transthoracic echocardiogram of the patient from case 1 showed a 20 × 20 mm sized myxoma in the right ventricular outflow tract. M: cardiac myxoma, RV: right ventricle, IVS: interventricular septum, LV: left ventricle, LA: left atrium, PA: pulmonary artery.
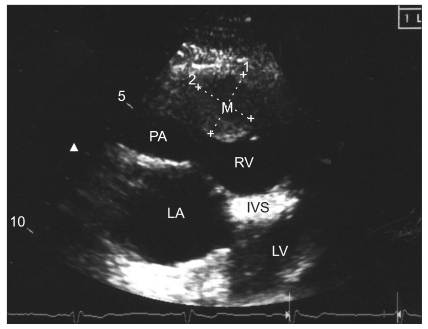
Fig. 2
Pre-operative chest computed tomogram of the patient from case 1 showed a myxoma in the main pulmonary trunk. M: cardiac myxoma.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download