Abstract
Background
Endoscopic thyroidectomy was recently introduced and has been rapidly accepted by surgeons and patients. The present study was conducted to estimate and compare the incidences of postoperative nausea and vomiting (PONV) after endoscopic thyroidectomy using two different anesthetic methods: sevoflurane based balanced anesthesia; total intravenous anesthesia (TIVA).
Methods
Ninety nine female patients that were scheduled to undergo elective endoscopic thyroidectomy under general anesthesia were enrolled. These patients were randomly allocated to receive sevoflurane based balanced anesthesia (BA group) or propofol-remifentanil anesthesia (TIVA group). PONV was evaluated using a 4-point Likert scale, and pain using a visual analogue scale (VAS; range 0 to 100) for 0-2, 2-6, and 6-24 hours postoperatively. At 24 hours postoperatively, overall patient satisfaction regarding PONV and pain were recorded.
Results
The incidence of PONV was 14.6% in the TIVA group and 51.3% in the BA group. The incidence of nausea at 0-2 and 2-6 hours postoperatively was lower in the TIVA group than in the BA group (4.2% vs. 35.9%, 6.3% vs. 23.1%, respectively), but no between-group difference was observed at 6-24 hours postoperatively (8.3% vs. 5.1%). Antiemetic usage at 0-2 and 2-6 hours was lower in the TIVA than the BA group (4.2% vs. 38.5%, 6.3% vs. 23.1%), but no between-group difference was observed for 6-24 hours (6.3% vs. 7.7%). There were no differences in pain or in patient satisfaction.
Postoperative nausea and vomiting (PONV) is one of the most distressing side effects, and thus is an important cause of patient dissatisfaction during the postoperative period [1]. In addition, PONV can lead to delayed post-anesthesia care unit (PACU) discharge, unanticipated hospital admission, and higher medical costs. Currently, the incidence of PONV is believed to be 25 to 73%, and the incidence of severe, intractable PONV to be approximately 0.2 to 8% among patients that undergo surgery [2-4].
Endoscopic thyroidectomy has been rapidly accepted by surgeons and patients because of its cosmetic advantages. Conventional open thyroidectomy is a PONV prone surgery with an incidence as high as 70% [5]. However, there appears to be an even greater risk of PONV after endoscopic thyroidectomy, because younger, female patients tend to choose endoscopic thyroidectomy over conventional open thyroidectomy and because laparoscopic surgery, CO2 insufflation, and longer surgery and anesthesia durations also are common features of endoscopic thyroidectomy. All of these characteristics are risk factors for PONV [3,4,6,7].
However, there have been no reports regarding PONV after endoscopic thyroidectomy. Thus, the present research was carried out to estimate the incidence of PONV after endoscopic thyroidectomy. In the present study, the incidence of PONV was compared between two anesthetic methods - sevoflurane based balanced anesthesia (BA) and total intravenous anesthesia (TIVA). In a previous open thyroidectomy study, propofol anesthesia was found to induce PONV less frequently than isoflurane anesthesia [5]. Therefore, we hypothesized that TIVA would show less PONV than sevoflurane based balanced anesthesia.
This prospective, double-blind, randomized study was approved by the Research Board at our hospital, and written informed consent was obtained from all patients.
Female patients that were scheduled to undergo elective endoscopic thyroidectomy under general anesthesia were considered for this study. Patients with a history of a respiratory or cardiovascular disease, smoking, PONV, motion sickness and those that had taken an antiemetic during the 24 hours period before surgery were excluded. Finally, 99 patients were enrolled in the present study.
Participants were randomly allocated to receive sevoflurane anesthesia with remifentanil co-administration (the BA group) or propofol-remifentanil anesthesia (the TIVA group) using a sealed envelope technique. Patients and designated observers of PONV were unaware of the anesthetic method given to any particular patient.
No patient received premedication. Intraoperative monitoring included noninvasive blood pressure (NIBP) monitoring, electrocardiography (ECG), pulse oximetry, and capnography. A blood pressure cuff was applied to the ankle to avoid disrupting the operative procedure. In the BA group, general anesthesia was induced using thiopental sodium 4-5 mg/kg and rocuronium 0.9 mg/kg. After intubation, anesthesia was maintained using sevoflurane (1-1.5 minimum alveolar concentration (MAC)) in 50% O2 with air. Remifentanil was co-administered as a continuous infusion and titrated to maintain a mean blood pressure within 20% of baseline. In the TIVA group, propofol and remifentanil were administered to end organ concentrations of 5.0 µg/ml and 4.0 ng/ml, respectively, using a target-controlled infusion (TCI) pump (Orchestra®, Fresenius Vial, France). Rocuronium 0.9 mg/kg was administered to facilitate intubation. Propofol and remifentanil infusions were titrated to maintain a mean blood pressure within 20% of baseline during anesthesia. O2 and air were administered in a 1 : 1 ratio.
All endoscopic thyroidectomies were performed by two experienced surgeons using the bilateral axillo-breast approach (BABA) with patients in the supine position with neck extension using a shoulder pillow [8]. Before making the skin incision, 150-200 ml of a 1 : 200,000 epinephrine solution was injected into the subcutaneous space in both breasts and in the subplatysmal space in the neck to reduce bleeding during dissection. After making 2 incisions on both upper circumareolar areas, subcutaneous and subplatysmal dissections were performed bluntly using a Rochester clamp and vascular tunneler. A 12-mm trocar was then placed ipsilateral to the thyroid mass to introduce a flexible endoscope. A contralateral 12-mm port was used for the operational instruments, which included a Harmonic scalpel. Carbon dioxide was then insufflated using a continuous pressure of 5-6 mmHg to prevent subcutaneous emphysema and reduce any minor bleeding.
Before the end of surgery, meperidine 0.5 mg/kg was injected for postoperative pain control and neuromuscular relaxation was reversed with pyridostigmine 15 mg and glycopyro-phosphate 0.4 mg in both groups. All patients were transferred to the PACU.
Blood pressures, SpO2 and EtCO2 values, end expiratory sevoflurane concentrations, end organ concentrations of remifentanil and propofol, and peak inspiratory pressures were recorded at baseline, 1 minute after intubation, just after CO2 insufflation, and subsequently at 10 minute intervals until the end of the procedures. Amount of intraoperative fluid, urine output, total amount of remifentanil and propofol, duration of anesthesia and duration of the procedure was also recorded.
A trained investigator visited each patient at 2, 6, and 24 hours postoperatively, and checked the occurrence and severity of PONV. Nausea was defined as the sensation of having the urge to vomit, and was graded using a 4-point Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). Vomiting was defined as the forceful evacuation of stomach contents. Ondansetron 4 mg or metoclopropamide 10 mg was administered after a patient vomited or when a patient requested an antiemetic; and these instances of drug administration were recorded.
Pain was evaluated at 2, 6, and 24 hours postoperatively using a visual analogue scale (VAS; range 0 to 100). If VAS was higher than 50 or a patient requested pain medication, ketorolac 30 mg was injected, and if this was ineffective, meperidine 0.5 mg/kg was added.
At 24 hours postoperatively, overall patient satisfaction regarding the treatment of PONV and pain were recorded as very good, good, fair, bad, or poor.
No report has been previously issued regarding the incidence of PONV after endoscopic thyroidectomy. However, the incidence of PONV after isoflurane anesthesia and propofol anesthesia for open thyroidectomy have been reported to be 47% and 24%, respectively [9]. Therefore, we considered a 30% reduction of PONV in the TIVA group versus the BA group as significant. To identify this 30% reduction with an alpha error of 0.05 and a power of 80%, the required sample size was calculated to be 39 per group. Initially, 99 patients were included in the study, assuming a dropout rate of 20%, and 87 completed the study (39 in the BA group, 48 in the TIVA group).
PONV and pain at 2, 6, and 24 hours postoperative were compared using the Mann-Whitney U-test or the Chi-square test. Statistical significance was accepted for P values of < 0.05.
Twelve of 99 patients were excluded from the analysis. In two, the operation method was changed to open thyroidectomy, in three, the anesthetic method was altered, and the other seven were excluded due to incomplete data. Therefore, the data of 87 patients were analyzed, 39 in the BA group and 48 patients in the TIVA group.
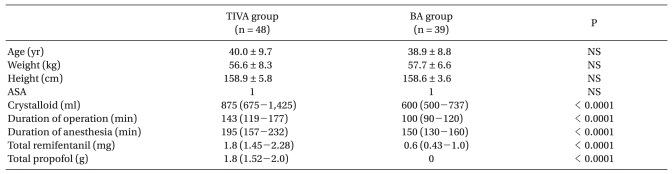
Age, weight, height, and ASA physical status were not significantly different in the two study groups. However, duration of surgery and anesthesia was longer in the TIVA group, differences which appeared to have been caused by a greater proportion of total thyroidectomy cases in the TIVA group (total thyroidectomy: 54.2% vs. 35.9%, hemithyroidectomy: 45.8% vs. 64.1%, in the TIVA and BA groups, respectively, P = 0.130). Total remifentanil used during surgery was greater in the TIVA group than in the BA group (Table 1).
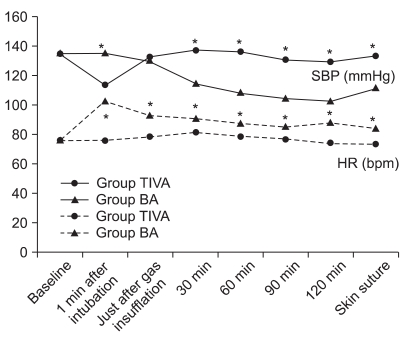
Systolic blood pressure (SBP) and heart rate at baseline were not significantly different between the two groups. SBP was significantly higher at 1 minute after intubation, but was maintained at a lower level during the operation in the BA group than in the TIVA group, and heart rate (HR) from the time of intubation to the end of surgery was significantly greater in the BA group (Fig. 1).
No significant difference in end-tidal CO2 (TIVA group = 33.0 ± 6.8 mmHg vs. the BA group = 32.6 ± 4.4 mmHg) or peak inspiratory pressure (the TIVA group = 17.9 ± 9.4 cm H2O vs. the BA group = 18 ± 9.4 cm H2O) was observed between the two groups.
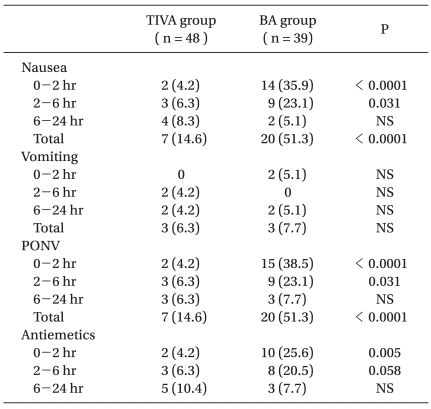
The overall incidence of PONV was 14.6% and 51.3% in the TIVA and BA groups (P < 0.05). The incidence of nausea was significantly lower in the TIVA group than in the BA group at 0-2 and 2-6 hours postoperatively (0-2 hours: 4.2% vs. 35.9%, 2-6 hours: 6.3% vs. 23.1%) but not at 6-24 hours postoperatively (8.3% vs. 5.1%, in the TIVA and BA groups, respectively). Antiemetic use showed a similar trend. No significant difference was observed between the two groups in terms of the incidence of vomiting at any postoperative time point (Table 2).
The severity of nausea, as measured using a 4-point Likert scale, showed that mild nausea predominated. The prevalence of mild nausea in the TIVA and BA groups was: 4.2% vs. 23.1% at 0-2 hours, 6.2% vs. 20.5% at 2-6 hours, and 6.2% vs. 5.1% at 6-24 hours, respectively (P > 0.05). The prevalence of moderate and severe nausea were, in the TIVA and BA groups, respectively: 0% vs. 12.8% at 0-2 hours, 0% vs. 2.6% at 2-6 hours, and 2.1% vs. 0% at 6-24 hours (P > 0.05).
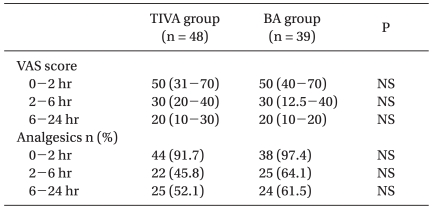
VAS scores and analgesic use were not significantly different in the two study groups (Table 3); neither was time spent in the PACU (66 ± 9 min for the TIVA group; 67 ± 12 min for the BA group).
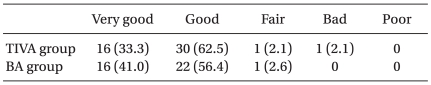
In both groups, almost all patients were satisfied with the treatment of PONV and pain (Table 4).
The incidence of PONV during the first 24 postoperative hours after endoscopic thyroidectomy were 14.6% and 51.3% in the TIVA and BA groups, respectively. The type of anesthesia was found to influence PONV significantly during the early postoperative period (0-6 hours), but not subsequently.
When we initiated this study, we anticipated a higher incidence of PONV after endoscopic thyroidectomy than that associated with open thyroidectomy. In particular, because laparoscopy with CO2 insufflation is known to increase PONV [10,11], it has been suggested that the etiology of PONV after laparoscopy is due to residual stretching and irritation of the peritoneum [10] or to the dilation of cerebral vessels and an increase in intracranial pressure (ICP) caused by the CO2 [11]. However, the incidence of PONV observed during the present study was similar to that reported for open thyroidectomy (~50%) for inhalation groups. In the case of propofol anesthesia, the PONV incidence was even lower (14.6%) than that after open thyroidectomy (24%)[9]. During endoscopic thyroidectomy, CO2 is applied between subcutaneous tissue and muscles rather than intraperitoneally, and the insufflation pressure is usually limited to 5-6 mmHg instead of 12 mmHg to avoid acidosis or adverse haemodynamic changes [10-12]. In addition, we modulated tidal volume and respiratory rate to maintain an EtCO2 of 35-45 mmHg, and therefore, the effect of CO2 insufflation on PONV after endoscopic thyroidectomy might not be as great as after laparoscopy. Further studies are required to compare open and endoscopic thyroidectomy with respect to the incidence of PONV.
The primary end point of our study was the incidence of PONV between the BA and TIVA groups. In previous open thyroidectomy studies, propofol anesthesia was found to reduce the incidence of PONV as compared with inhalational anesthesia [5,9]. Gauger and colleagues also found that PONV was significantly less likely in a propofol group during the early period (in the operating room and postanesthesia care unit) but not at later times (postoperative day 1 or 2) [9]. The results of these two open thyroidectomy studies are in line with the results of our endoscopic thyroidectomy study. In our study, the early postoperative PONV incidence was lower in the TIVA group than in the BA group.
Propofol is known to have an antiemetic effect, and the use of propofol during balanced or total intravenous anesthesia had been reported to significantly reduce the incidence of opioid-induced nausea and vomiting [13]. Some authors have reported that propofol used as a maintenance anesthetic during breast surgery is more effective than 4 mg of ondansetron given as prophylaxis in terms of preventing PONV [14]. The exact mechanism underlying the antiemetic effect of propofol has not been determined, but it has been reported that propofol does not interact strongly with D2 dopamine receptors [15].
In the present study, a greater amount of remifentanil was administered in the TIVA group, but the incidence of PONV was much lower in this group than in the BA group. Although chemically related to fentanyl congeners, remifentanil is structurally unique because of its ester linkages. This ester group in remifentanil renders it susceptible to hydrolysis by blood and tissue nonspecific esterases. which results in its rapid metabolism and a reduction in its blood concentrations after infusion is terminated [16,17]. Therefore, the higher amount of remifentanil administered in the TIVA group might not have increased PONV due to its rapid elimination.
Other factors that are known to influence PONV, such as pain and postoperative analgesic consumption, did not differ between the two study groups. Furthermore, operation and anesthesia duration, which are known to influence PONV, were greater in the TIVA group. Thus, had operation and anesthesia duration been similar, the two groups would probably have differed more in terms of PONV.
In the present study, the volume of infused crystalloid solution was greater in the TIVA group. This was because the TIVA group had a longer operating time than the BA group. The impact of the volume of crystalloid on PONV is not well defined. In a recent study, Dagher et al. failed to observe any benefit of rapid infusion of 30 ml/kg over 10 ml/kg of crystalloid solution in reducing the incidence of PONV after thyroidectomy [18]. Therefore, the difference in volume of crystalloid solution probably had no effect on PONV in either group.
In the present study, the incidence of vomiting was not different between the two groups, which may have been because we administered antiemetics as soon as patients complained of nausea or requested antiemetics, and the incidence of antiemetic administration was significantly higher in the BA group.
The TIVA group was found to have higher systolic blood pressure than the BA group during the operation. It has been reported that during laparoscopic oophorectomy, more stress hormones are released during TIVA anesthesia than during sevoflurane anesthesia [19], and thus, the higher stress hormone levels observed in the TIVA group could explain the higher systolic pressure. Alternatively, the higher SBP could have been due to the fact that 150-200 ml of 1 : 200,000 epinephrine is routinely infiltrated during endoscopic thyroidectomy, and the long lasting hemodynamic effect of slowly absorbed epinephrine might not have been counterbalanced as effectively as sevoflurane during TIVA. Propofol is administered in much lower doses when co-administered with remifentanil. Opioids have fewer cardiovascular effects than many other intravenous and inhaled anesthetics. Furthermore, opioids reverses blood pressure elevation mainly by suppressing the central release of stress hormones, and, therefore, they have little ability to antagonize externally administered epinephrine [20]. Further detailed comparative studies on hemodynamic changes during endoscopic thyroidectomy associated with BA and TIVA are required.
In conclusion, the present study shows that the incidence of PONV after sevoflurane-based balanced anesthesia during endoscopic thyroidectomy is as high as 50%. TIVA with propofol-remifentanil was found to reduce the incidence of PONV to 15% and its antiemetic effect was found to be maintained for 6 hours after surgery.
References
1. Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000; 84:6–10. PMID: 10740539.
2. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000; 59:213–243. PMID: 10730546.

3. Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997; 52:443–449. PMID: 9165963.

4. Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994; 78:7–16. PMID: 8267183.
5. Sonner JM, Hynson JM, Clark O, Katz JA. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth. 1997; 9:398–402. PMID: 9257207.

6. Choi DH, Ko JS, Ahn HJ, Kim JA. A Korean predictive model for postoperative nausea and vomiting. J Korean Med Sci. 2005; 20:811–815. PMID: 16224155.

7. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004; 350:2441–2451. PMID: 15190136.

8. Choe JH, Kim SW, Chung KW, Park KS, Han W, Noh DY, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007; 31:601–606. PMID: 17308853.

9. Gauger PG, Shanks A, Morris M, Greenfield ML, Burney RE, O'Reilly M. Propofol decreases early postoperative nausea and vomiting in patients undergoing thyroid and parathyroid operations. World J Surg. 2008; 32:1525–1534. PMID: 18305999.

10. Iitomi T, Toriumi S, Kondo A, Akazawa T, Nakahara T. Incidence of nausea and vomiting after cholecystectomy performed via laparotomy or laparoscopy. Masui. 1995; 44:1627–1631. PMID: 8583657.
11. Koivusalo AM, Kellokumpu I, Lindgren L. Postoperative drowsiness and emetic sequelae correlate to total amount of carbon dioxide used during laparoscopic cholecystectomy. Surg Endosc. 1997; 11:42–44. PMID: 8994987.

12. Bellantone R, Lombardi CP, Rubino F, Perilli V, Sollazzi L, Mastroianni G, et al. Arterial PCO2 and cardiovascular function during endoscopic neck surgery with carbon dioxide insufflation. Arch Surg. 2001; 136:822–827. PMID: 11448398.

13. Raftery S, Sherry E. Total intravenous anaesthesia with propofol and alfentanil protects against postoperative nausea and vomiting. Can J Anaesth. 1992; 39:37–40. PMID: 1531118.

14. Gan TJ, Ginsberg B, Grant AP, Glass PS. Double-blind, randomized comparison of ondansetron and intraoperative propofol to prevent postoperative nausea and vomiting. Anesthesiology. 1996; 85:1036–1042. PMID: 8916820.

15. Appadu BL, Strange PG, Lambert DG. Does propofol interact with D2 dopamine receptors? Anesth Analg. 1994; 79:1191–1192. PMID: 7978445.

16. Westmoreland CL, Hoke JF, Sebel PS, Hug CC Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. 1993; 79:893–903. PMID: 7902033.

17. Ozkose Z, Yalcin Cok O, Tuncer B, Tufekcioglu S, Yardim S. Comparison of hemodynamics, recovery profile, and early postoperative pain control and costs of remifentanil versus alfentanil-based total intravenous anesthesia (TIVA). J Clin Anesth. 2002; 14:161–168. PMID: 12031745.

18. Dagher CF, Abboud B, Richa F, Abouzeid H, El-Khoury C, Doumit C, et al. Effect of intravenous crystalloid infusion on postoperative nausea and vomiting after thyroidectomy: a prospective, randomized, controlled study. Eur J Anaesthesiol. 2009; 26:188–191. PMID: 19237980.

19. Marana E, Scambia G, Colicci S, Maviglia R, Maussier ML, Marana R, et al. Leptin and perioperative neuroendocrine stress response with two different anaesthetic techniques. Acta Anaesthesiol Scand. 2008; 52:541–546. PMID: 18339160.

20. Myre K, Raeder J, Rostrup M, Buanes T, Stokland O. Catecholamine release during laparoscopic fundoplication with high and low doses of remifentanil. Acta Anaesthesiol Scand. 2003; 47:267–273. PMID: 12648191.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download