Abstract
Background
Microemulsion propofol produces more frequent and severe pain upon injection than lipid emulsion propofol. This study examined the analgesic effect of lidocaine-premixed microemulsion propofol in patients pretreated with remifentanil. The induction of anesthesia with this combination was compared with microemulsion propofol accompanied with either remifentanil or lidocaine.
Methods
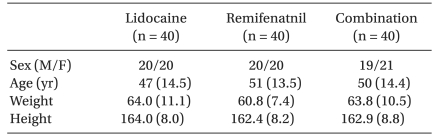
One hundred twenty patients aged between 20-65 years old were allocated randomly into one of three groups (n = 40, in each). The patients in the remifentanil group received remifentanil 0.5 µg/kg IV for 30 seconds before a microemulsion propofol injection. The patients in the lidocaine group received propofol 2 mg/kg premixed with 40 mg lidocaine over a 60 second period. The patients in the combination group received both remifentanil and lidocaine.
Results
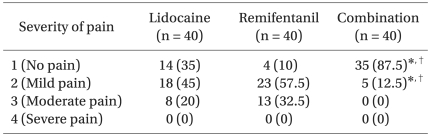
There was a significantly lower incidence of microemulsion propofol injection pain (severity 2 or more) in the combination group (12.5%) than in the remifentanil and lidocaine groups (90% and 65%, respectively, P < 0.05). The incidence of moderate pain disappeared completely in the combination group (0%) compared to that in the remifentanil and lidocaine group (32.5% and 20%, respectively, P < 0.05). Severe pain did not appear in any of the three groups. There were no complications on the injection site in the lidocaine alone and combination groups.
Despite its many advantages, propofol is also associated with some anesthetic challenges, such as difficulty in developing an injectable formulation, significant decreases in blood-pressure at a normal induction dose, and prominent pain at the peripheral intravenous injection sites [1]. Those problems are associated with the currently used long-chain triglyceride formulations but may provide further pharmaceutical opportunity to develop a newer and better generation of propofol.
A lipid-free microemulsion propofol (Aquafol®; Daewon Pharmaceutical Co., Ltd, Seoul, Korea), which is composed of 1% propofol, 10% purified poloxamer 188 (PP188) as a nonionic block copolymer surfactant and 0.7% polyethylene glycol 660 hydroxystearate as a nonionic surfactant, was developed to avoid the risk of adverse lipid solvent-related drug reactions, such as fat embolism, postoperative infection, hypertriglyceridaemia and pancreatitis [2], However, microemulsion propofol produces more frequent and severe pain upon injection than long-chain triglyceride propofol [3].
Many techniques have been suggested to prevent such pain with varying degrees of success. These include premedication [4], rapid injection [5], dilution or changing the temperature of propofol [6], use of local anesthetics [7-9], and pre-treatment with systemic opioids [10-12]. However, none has achieved the complete elimination of pain. Recent studies revealed that a combination of two different analgesic modalities, opioids and lidocaine, can reduce the incidence and severity of propofol injection pain compared to each drug alone in adults [13,14].
This study examined the analgesic effect of microemulsion propofol premixed with lidocaine after a pretreatment with remifentanil, and compared the effect with that of each treatment alone.
After obtaining approval from the institutional review board and informed consent, the study was carried out prospectively on 120 ASA (American Society of Anesthesiologists) physical status I or II patients aged between 20-65 years, who underwent general anesthesia for elective surgery. Patients with self-confirming allergies to opioids, local anesthetics, asthma, neurological deficits and those who had received analgesics or sedatives within the previous 24 hours were excluded.
No premedication was administered prior surgery. Before arriving at the operating room, a 20 gauge cannula was inserted in the left cephalic vein, and its position was confirmed by the free flow of a Hartmann's solution infused by gravity. Upon arrival at the operating room, all patients were monitored with an electrocardiogram, pulse oximeter and non-invasive arterial pressure.
The patients were allocated randomly to one of three groups using a computer generated randomization list manipulated by a statistician in a sealed envelope. An independent researcher prepared the study syringe for each patient. The patients' characteristics were similar in the three groups (Table 1). No patient was excluded from the analysis due to complications, hence the data for all 120 patients is presented. Regarding the treatment groups, the remifentanil group received remifentanil 0.5 µg/kg intravenously (diluted with normal saline) for 30 s and at 60 s later patients were given 2 mg/kg microemulsion propofol for 60 s. The lidocaine group received 2 mg/kg microemulsion propofol premixed with lidocaine 40 mg over a 60 s period. The combination group received remifentanil 0.5 µg/kg IV (diluted with normal saline) over a 30 s period, and 60 s later, the patients were given microemulsion propofol premixed with lidocaine 40 mg over a 60 s period. After the remifentanil injection, the Observer's Assessment of Alertness and Sedation (OAA/S) scale was checked to subjectively assess the level of consciousness to ensure an adequate response to the pain questionnaires [15]. The mean arterial pressure and heart rate were recorded before injecting the study drug (baseline), after the remifentanil injection, after the microemulsion propofol injection and 1 minute after tracheal intubation.
The patients, anesthesia providers and investigators who scored the movements were blinded to the treatment group. All study drugs were prepared before the injection at room temperature. Microemulsion propofol (Aquafol®, 1% propofol, Daewon Pharmaceutical Co., Ltd, Seoul, Korea) was mixed with 2 ml of lidocaine 2% (or normal saline 2 ml). All drugs were administered through a rubber port connected to the intravenous cannula with a free flow of fluid. After preoxygenation, general anesthesia was induced with 2 mg/kg microemulsion propofol. Mask ventilation was initiated with oxygen 100% once the patient had become unconscious and apneic. The patients' response after the microemulsion propofol injection was graded by the investigator according to the following four-point scale, as previously described [16]: 1, no pain (no reaction to the injection); 2, slight pain (a minor verbal/facial response or motor reaction to the injection); 3, moderate pain (a clear verbal/facial response or motor reaction to the injection); and 4, severe pain (the patient both complained of pain and withdrew their arm).
The assessment was made from the start of the microemulsion propofol injection to the point when the patients had lost consciousness. The investigator also recorded the incidence of cough, chest rigidity and breath holding. After the loss of an eyelash reflex, the patients were intubated after administering rocuronium 0.8 mg/kg. Anesthesia was maintained with sevoflurane 2.0% to 2.5% and nitrous oxide 50% in oxygen.
The sample size calculation was based on preliminary data. In a one-way ANOVA study, sample sizes of 37, 37, and 37 were obtained for the 3 groups, whose means of incidence were to be compared. The total sample of 111 subjects was found to be sufficient to achieve 80% power to detect differences between the means versus the alternative of equal means using an F test with a 0.05 significance level. The size of the variation in the means is represented by their standard deviation, 15.05. The common standard deviation within a group was assumed to be 50. The sample size was increased to 40 patients per group assuming the occurrence of dropouts.
Statistical analyses were performed using SPSS software (version 12.0, SPSS Inc., IL, USA). A Fisher's exact test was used to calculate the between-group differences in the incidence of microemulsion-induced pain, and a Kruskal-Wallis test was used to assess the differences in the mean pain-intensity scores. P value < 0.05 was considered significant. All values are expressed as the mean (SD) or absolute numbers (%).
The incidence of pain from a microemulsion propofol injection (severity 2 or more) was significantly lower in the combination group (12.5%) than that in the remifentanil and lidocaine groups (90% and 65%, respectively, P < 0.05) (Table 2). The incidence of moderate pain disappeared completely in the combination group (0%), compared to that in the remifentanil and lidocaine groups (32.5% and 20%, respectively, P < 0.05).
No case of severe pain was observed in any of the three groups (Table 2). For all subjects, the OAA/S levels were 5 (prompt response to name spoken in a normal tone), indicating adequate responses to the questionnaires.
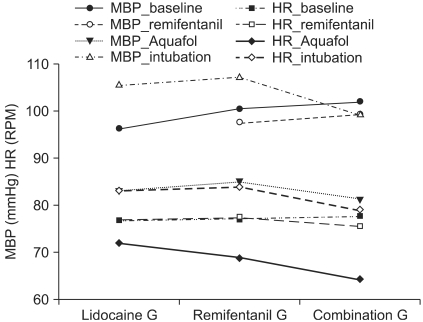
The heart rate (HR) and mean arterial pressure (MAP) were maintained within the normal limits in all three groups and there was no hypotension or bradycardia encountered during the study period (Fig. 1). None of the patients suffered from desaturation, apnea, chest wall rigidity, cough or other adverse effects during the induction of anesthesia. Only three patients in the remifentanil group had a little wheal reaction on the injection site but those responses disappeared within a few minutes.
Propofol-induced pain has been ranked by American anesthesiologists as the seventh most important problem of current clinical anesthesiology [17]. The nature of pain is extreme aching, burning and crushing. Pain during propofol injection can be immediate or delayed, and delayed pain, which is has been attributed to an interaction with nociceptors and free nerve endings, has a latency of between 10 and 20 seconds [18].
This study demonstrated that a combination of a pretreatment with remifentanil 0.5 µg/kg and premixture of 40 mg lidocaine and microemulsion propofol was more effective in reducing the incidence of pain on an injection of microemulsion propofol than each treatment alone.
The more frequent and severe pain of the microemulsion propofol injection than that of lipid emulsion propofol is a common and difficult problem. A recent study demonstrated that the incidence of pain (VAS > 30 mm) on injection with microemulsion and lipid emulsion propofol was 69.7% and 42.3%, respectively, and the median (25%, 75%) VAS scores for pain on injection with microemulsion and lipid emulsion propofol were 59 (25, 85) and 24 (0, 50) mm, respectively. The significantly higher incidence and severity of pain on injection with microemulsion propofol are associated with a sevenfold increase in the aqueous free propofol concentration [3].
The most popular technique for reducing the injection pain of propofol is to mix lidocaine with propofol [9]. Lidocaine may act by stabilizing the kinin cascade [7], which is activated by contact with free propofol [19]. The analgesic effect of lidocaine on a propofol injection is based not only on its local anesthetic effects, but also on the decrease in pH of the propofol-lidocaine mixture [20].
Pretreatment with opioids has been reported to reduce the incidence and severity of pain during a propofol injection with varying results [10-12]. Remifentanil is a piperidine-based opioid that acts as a µ-receptor agonist. Its pharmacokinetic profile is unique among opioids with very rapid plasma clearance and onset time and a very short context-sensitive half-life of 2-10 min. Therefore, remifentanil appears to be a very titratable opioid providing profound intraoperative analgesia for either very brief periods in which analgesia is required or over prolonged periods without any concern for prolonged recovery [21]. Similar to other opioids, the action site of remifentanil may either be central or peripheral. Our assumption was that the pain-reducing action of remifentanil would mainly be central because a tourniquet technique was not used and adequate time was allowed for the onset of remifentanil.
In this study, the injection pain of microemulsion propofol was reduced to 12.5% of patients in the combination group. In contrast, 65-90% of patients in the lidocaine and remifentanil groups suffered from a painful injection. These results suggest that remifentanil enhances the analgesic efficacy of the lidocaine premixture. Further study elucidating the mechanism of this effect is needed.
Although the decrease in HR and MAP before intubation was statistically significant in the remifentanil and combination groups, the MAP and HR before intubation were maintained within the normal limits (variation < 20%). This study had some limitations. First, the sample size was relatively small despite the sufficient number of patients according to power analysis. Second, a non-treated control group was not included in this study.
In conclusion, the combination treatment of two different analgesic modalities, remifentanil and lidocaine, prevents the moderate and severe pain on microemulsion propofol injection, and reduces the incidence of mild pain compared to each drug alone.
References
1. Baker MT, Naguib M. Propofol. The challenges of formulation. Anesthesiology. 2005; 103:860–876. PMID: 16192780.
2. Kim KM, Choi BM, Park SW, Lee SH, Christensen LV, Zhou J, et al. Pharmacokinetics and pharmacodynamics of propofol microemulsion and lipid emulsion after an intravenous bolus and variable rate infusion. Anesthesiology. 2007; 106:924–934. PMID: 17457123.

3. Sim JY, Lee SH, Park DY, Jung JA, Ki KH, Lee DH, et al. Pain on injection with microemulsion propofol. Br J Clin Pharmacol. 2009; 67:316–325. PMID: 19220277.

4. Fragen RJ, de Grood PM, Robertson EN, Booij LH, Crul JF. Effects of premedication on diprivan induction. Br J Anaesth. 1982; 54:913–916. PMID: 6981419.
5. Rolly G, Versichelen L, Huyghe L, Mungroop H. Effect of speed of injection on induction of anaesthesia using propofol. Br J Anaesth. 1985; 57:743–746. PMID: 3874643.

6. McCrirrick A, Hunter S. Pain on injection of propofol: the effect of injectate temperature. Anaesthesia. 1990; 45:443–444. PMID: 2200300.

7. Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988; 43:492–494. PMID: 3261547.

8. Mangar D, Holak EJ. Tourniquet at 50 mmHg followed by intravenous lidocaine diminishes hand pain associated with propofol injection. Anesth Analg. 1992; 74:250–252. PMID: 1731546.
9. Gehan G, Karoubi P, Quinet F, Leroy A, Rathat C, Pourriat JL. Optimal dose of lignocaine for preventing pain on injection of propofol. Br J Anaesth. 1991; 66:324–326. PMID: 2015149.

10. Roehm KD, Piper SN, Maleck WH, Boldt J. Prevention of propofol-induced injection pain by remifentanil: a placebo-controlled comparison with lidocaine. Anaesthesia. 2003; 58:165–170. PMID: 12625310.
11. Basaranoglu G, Erden V, Delatioglu H, Saitoglu L. Reduction of pain on injection of propofol using meperidine and remifentanil. Eur J Anaesthesiol. 2005; 22:890–892. PMID: 16225729.

12. Iyilikci L, Balkan BK, Gokel E, Gunerli A, Ellidokuz H. The effects of alfentanil or remifentanil pretreatment on propofol injection pain. J Clin Anesth. 2004; 16:499–502. PMID: 15590252.

13. Kwak K, Kim J, Park S, Lim D, Kim S, Baek W, et al. Reduction of pain on injection of propofol: combination of pretreatment of remifentanil and premixture of lidocaine with propofol. Eur J Anaesthesiol. 2007; 24:746–750. PMID: 17261216.

14. Aouad MT, Siddik-Sayyid SM, Al-Alami AA, Baraka AS. Multimodal analgesia to prevent propofol-induced pain: pretreatment with remifentanil and lidocaine versus remifentanil or lidocaine alone. Anesth Analg. 2007; 104:1540–1544. PMID: 17513655.

15. Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990; 10:244–245. PMID: 2286697.
16. King SY, Davis FM, Wells JE, Murchison DJ, Pryor PJ. Lidocaine for the prevention of pain due to injection of propofol. Anesth Analg. 1992; 74:246–249. PMID: 1731545.

17. Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999; 88:1085–1091. PMID: 10320175.

18. Briggs LP, Clarke RS, Dundee JW, Moore J, Bahar M, Wright PJ. Use of di-isopropyl phenol as main agent for short procedures. Br J Anaesth. 1981; 53:1197–1202. PMID: 6976790.

19. Dubey PK, Kumar A. Pain on injection of lipid-free propofol and propofol emulsion containing medium-chain triglyceride: a comparative study. Anesth Analg. 2005; 101:1060–1062. PMID: 16192520.

20. Eriksson M, Englesson S, Niklasson F, Hartvig P. Effect of lignocaine and pH on propofol-induccd pain. Br J Anaesth. 1997; 78:502–506. PMID: 9175962.
21. Glass PS, Gan TJ, Howell S. A review of the phamacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999; 89:7–14. PMID: 10389771.
Fig. 1
Hemodynamic changes after the propofol and remifentanil injection. The blood pressure and heart rate are the mean values that were maintained within the normal limits in all three groups and there was no hypotension or bradycardia during the study period. MBP baseline: MBP before injecting the study drug, MBP remifentanil: MBP after the remifentanil injection, MBP Aquafol: MBP after the Aquafol injection, MBP intubation: MBP 1 min after intubation. HR baseline: baseline HR, HR remifentanil: HR after the remifentanil injection, HR Aquafol: HR after the Aquafol injection, HR intubation: HR 1 min after intubation. MBP: mean blood pressure, HR: heart rate.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download