Abstract
Diabetic nephropathy is the most common cause of end-stage renal disease and accounts for significant morbidity and mortality among individuals with diabetes mellitus. Therefore, the clarification of the pathogenesis of diabetic nephropathy is an urgent issue. Podocytes cover the outer layer of the glomerulus and maintain its integrity so that fluid and toxins exit in urine, but cells and important proteins are kept in the blood stream. Diabetes mellitus alters this structure, it becomes scarred and then the ability of the kidney to clear toxins is lost. Recent evidence shows that early in diabetes the podocyte number is reduced, areas of the glomerular basement membrane are denuded, and podocyte number predicts long-term urinary albumin excretion in the patients with diabetes and microalbuminuria. These results suggest that podocytes play a critical role in the early stage of diabetic nephropathy. It is the purpose of this article to review the pathogenetic role of podocytes in diabetic nephropathy.
Figures and Tables
Fig. 1
The glomerular urinary filtration barrier in normal non-diabetic rat. Representative photograph of electron microscopy shows three layers of urinary filtration barrier, the fenestrated glomerular endothelium (E), the glomerular basement membrane (BM), and the slit diaphragm of the podocyte. Magnification, × 40,000 [Permission from Lee et al. (reference 4)].
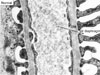
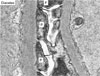
Fig. 2
Podocyte injury in diabetic rats. Representative electron photomicrographs show examples of effaced podocytes (P) and decreased number of slit pores (arrows) in the diabetic rats. Magnification, × 40,000 [Permission from Lee et al. (reference 4)].
References
2. Atlas of End-Stage Renal Disease in the United States. US Renal Data System USRDS 2007 Annual Data Report. 2007. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
3. Michaud JL, Kennedy CR. The podocyte in health and disease: insights from the mouse. Clin Sci. 2007. 112:325–323.
4. Lee EY, Lee MY, Hong SW, Chung CH, Hong SY. Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med J. 2007. 48:847–855.
5. Wang A, Ziyadeh FN, Lee EY, Pyagay PE, Sung SH, Sheardown SA, Laping NJ, Chen S. Interference with TGF-β signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol. 2007. 293:F1657–F1665.
6. Bjorn SF, Bangstad HJ, Hanssen KF, Nyberg G, Walker JD, Viberti GC, Osterby R. Glomerular epithelial foot processes and filtration slits in IDDM patients. Diabetologia. 1995. 38:1197–1204.
7. Ellis EN, Steffes MW, Chavers B, Mauer SM. Observations of glomerular epithelial cell structure in patients with type I diabetes mellitus. Kidney Int. 1987. 32:736–741.
8. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997. 99:342–348.
10. Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999. 42:1341–1344.
12. Lee EY, Chung CH, Kim JH, Joung HJ, Hong SY. Antioxidants ameliorate the expression of vascular endothelial growth factor mediated by protein kinase C in diabetic podocytes. Nephrol Dial Transplant. 2006. 21:1496–1503.
13. Lee EY, Shim MS, Kim MJ, Hong SY, Shin YG, Chung CH. Angiotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med. 2004. 36:65–70.
15. Mitu GM, Wang S, Hirschberg R. BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am J Physiol Renal Physiol. 2007. 293:F1641–F1648.
16. Kim MJ, Lee EY, Lee MY, Chung CH. Adipobiology of diabetes mellitus. Immunology, Endocrine and Metabolic Agents in Medicinal Chemistry. 2007. 7:123–127.
17. Lee EY, Chung CH, Wang A, Pyagay P, Chen S. Podocyte-derived MCP-1 is stimulated by TGF-β and might contribute to diabetic nephropathy. J Am Soc Nephrol. 2007. 18:655A–656A. (abstract).
18. Nakamura T, Ushiyama C, Shimada N, Sekizuka K, Ebihara I, Hara M, Koide H. Effect of the antiplatelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care. 2000. 23:1168–1171.
21. Gu L, Ni Z, Qian J, Tomino Y. Pravastatin inhibits carboxymethyllysine-induced monocyte chemoattractant protein 1 expression in podocytes via prevention of signaling events. Nephron Exp Nephrol. 2007. 106:e1–e10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download