Abstract
Background
Methods
Results
Figures and Tables
Fig. 1
Differences in the proportions of the subjects with (A) prediabetes and (B) diabetes among the five groups classified according to serum cystatin C levels. Serum cystatin C groups: first (<0.8 mg/L), second (0.8 mg/L), third (0.9 mg/L), fourth (1.0 mg/L), and fifth (>1.0 mg/L). aP values were calculated using the chi-square test.
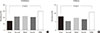
Table 1
The characteristics of the subjects with normal glucose, prediabetes, and diabetes defined by HbA1c levels

Values are presented as mean±standard deviation unless other indicated. P values were calculated using one-way analysis of variance analysis followed by post hoc testing with the S-N-K test unless otherwise indicated.
HbA1c, glycosylated hemoglobin; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood sugar; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; BUN, blood urea nitrogen; hs-CRP, high sensitivity C-reactive protein.
a,b,cThe same letters indicate insignificant differences between groups, dKruskal-Wallis test and Dunn's multiple comparison test.
Table 2
Differences in the parameter values among the five groups classified according to the range of serum cystatin C levels

Values are presented as mean±standard deviation unless other indicated. P values were calculated by one-way analysis of variance analysis followed by post hoc testing with the S-N-K test unless otherwise indicated.
WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; FBG, fasting blood sugar; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; BUN, blood urea nitrogen; hs-CRP, high sensitivity C-reactive protein.
a,b,c,d,eThe same letters indicate insignificant differences between groups, fSerum cystatin C groups: first (<0.8 mg/L), second (0.8 mg/L), third (0.9 mg/L), fourth (1.0 mg/L), and fifth (>1.0 mg/L), gKruskal-Wallis test and Dunn's multiple comparison test.
Table 3
OR (95% CI) for the prevalence of diabetic conditions

OR, odds ratio; CI, confidence interval.
aSerum cystatin C groups: first (<0.8 mg/L), second (0.8 mg/L), third (0.9 mg/L), fourth (1.0 mg/L), and fifth (>1.0 mg/L), bNon-adjusted model, cAge-adjusted (in total group, age- and sex-adjusted) model, dMultivariable model (additionally adjusting for body mass index, total cholesterol, and systolic blood pressure).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download