Abstract
Background
The waist-to-height ratio (WHtR) is an easy and inexpensive adiposity index that reflects central obesity. In this study, we examined the association of baseline WHtR and progression of coronary artery calcification (CAC) over 4 years of follow-up in apparently healthy Korean men.
Methods
A total of 1,048 male participants (mean age, 40.9 years) in a health-screening program in Kangbuk Samsung Hospital, Seoul, Korea who repeated a medical check-up in 2010 and 2014 were recruited. Baseline WHtR was calculated using the value for the waist in 2010 divided by the value for height in 2010. The CAC score (CACS) of each subject was measured by multi-detector computed tomography in both 2010 and 2014. Progression of CAC was defined as a CACS change over 4 years greater than 0.
Results
During the follow-up period, progression of CAC occurred in 278 subjects (26.5%). The subjects with CAC progression had slightly higher but significant baseline WHtR compared to those who did not show CAC progression (0.51±0.04 vs. 0.50±0.04, P<0.01). The proportion of subjects with CAC progression significantly increased as the baseline WHtR increased from the 1st quartile to 4th quartile groups (18.3%, 18.7%, 28.8%, and 34.2%; P<0.01). The risk for CAC progression was elevated with an odds ratio of 1.602 in the 4th quartile group of baseline WHtR even after adjustment for confounding variables (95% confidence interval, 1.040 to 2.466).
Abdominal obesity is a strong risk factor for the development of diabetes and cardiovascular diseases (CVD) [12]. Various anthropometric measures have been proposed to reflect adiposity, the most frequently used of which is body mass index (BMI). However, BMI does not take body fat distribution into account and it has limitations because fat distribution differs according to age, sex, and ethnicity [3]. Waist circumference (WC) and waist-hip ratio (WHR) have been used to discriminate visceral adiposity from simple obesity. However, WC also has limitations due to limited accounting for differences in height, which could lead to overestimation or underestimation in extremely tall or short individuals. Moreover, the WHR would mask the status of central obesity by an increase in hip circumference, and would be affected by the measurement errors of WC and hip circumference [4].
Waist-to-height ratio (WHtR) is an alternative measurement for visceral fat. A systematic review published in 2010 concluded that WHtR might be advantageous because it avoids the need for age-, sex-, and ethnicity-specific values [5]. The result of this systemic review found that a WHtR cutoff value of ≥0.5 identified people with increased risk for CVD and diabetes.
The coronary artery calcium score (CACS) is a marker for atherosclerosis and reflects the total atherosclerotic plaque burden at autopsy [67]. Previous studies have demonstrated a significant correlation between CACS and the risk of future CVD development and various metabolic diseases [891011]. In addition, large population-based cohort studies have reported a significant concordance between coronary artery calcification (CAC) prevalence and CVD risk strata assessed by the Framingham risk score [1213]. The use of CACS as a simple and safe surrogate marker for subclinical atherosclerosis is increasing.
In this study, we retrospectively examined the association of WHtR with the progression of CAC over 4 years in apparently healthy male participants in a health-screening program.
This was a retrospective longitudinal study and a part of the Kangbuk Samsung Health Study, which included participants in a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. The purpose of medical health checkup programs is to promote the health of employees through regular health checkups and to enhance early detection of existing diseases. Most of the examinees are employees and family members of various industrial companies from around the country. The employers largely pay for the costs of the medical examinations, and a considerable proportion of examinees undergo examinations annually or biannually.
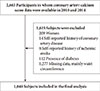
The initial study population was 2,663 subjects who participated in the medical checkup program between January 2010 and December 2010 and underwent CACS measurement at baseline, and repeated the medical checkup program and CACS measurement between January 2014 and December 2014. Of these subjects, 1,477 subjects were excluded owing to female gender (n=209), presence of self-reported history of coronary artery disease (n=14), ischemic stroke (n=8), diabetes (n=112), and missing data (n=1,277), resulting in 1,048 subjects for final analysis (Fig. 1). As 92.2% of the original study participants were men, final analyses were performed only in male participants.
The Institutional Review Board of Kangbuk Samsung Hospital approved this study. The requirement for informed consent was waived because we used unidentified data routinely collected during the health screening process.
Height and weight were measured twice and then averaged. The WHtR was calculated as the WC (cm) divided by the height (cm). The BMI was calculated by dividing the weight (kg) by the square of the height (m). The WC was measured in the standing position, at the middle point between the anterior iliac crest and the lower border of the rib, by a single examiner. Blood pressure was measured twice using a standardized sphygmomanometer after 5 minutes of rest and then averaged.
All subjects were examined after an overnight fast. The hexokinase method was used to determine the fasting glucose concentrations (Hitachi Modular D2400; Roche, Tokyo, Japan). Fasting serum insulin concentrations were determined by electrochemiluminescence immunoassay using a Hitachi Modular E170 (Roche). An enzymatic calorimetric test was used to measure the serum lipid profiles. Glycosylated hemoglobin (HbA1c) was measured by an immunoturbidimetric assay with a Cobra Integra 800 automatic analyzer (Roche Diagnostics, Basel, Switzerland) with a reference value of 4.4% to 6.4%. The methodology was aligned with the Diabetes Control and Complications Trial and National Glycohemoglobin Standardization Program (NGSP) standards [14]. The intra-assay coefficient of variation (CV) was 2.3% and the interassay CV was 2.4%, both within NGSP acceptable limits [15].
All subjects with a history of diabetes mellitus at baseline were excluded from the study. The presence of diabetes mellitus was determined by only the self-questionnaires completed by the participants, asking whether they had been diagnosed as diabetic or not. A current smoker was defined by responding 'yes' to the question 'Are you currently smoking?'
Multi-detector computed tomography (MDCT) for coronary calcium scoring was performed using a 64-slice, spiral computed tomography scanner (GE Health Care, Tokyo, Japan) with HEARTBEAT-CS software (Philips, Cleveland, OH, USA). Sixty-four-slice MDCT was performed using the following parameters: 0.625 mm slice thickness, 120 kVP, 800 effective mAs, and a 400 msec rotational speed. The severity of CAC was assessed by the Agatston score [16]. The total CACS was defined as the sum of the individual scores for the four major epicardial coronary arteries: left main, left anterior descending, left circumflex, and right coronary. The technicians who performed the MDCT were blinded to all patient information, and CACS was automatically calculated using the HEARTBEAT-CS software.
The data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Comparisons of baseline characteristics between those with or without CAC progression were analyzed using Student t-test and the percentages and prevalences were compared with the chi-square test.
The analyses for receiver operating characteristic (ROC) curves were performed to compare area under the curve (AUC) among baseline WHtR, BMI, and WC for the prediction of CAC progression.
Subjects were divided into four groups according to baseline WHtR as follows: quartile 1, <0.471; quartile 2, 0.471 to 0.497; quartile 3, 0.498 to 0.527; quartile 4, >0.527. Multivariate logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals of CAC progression according to quartile groups of baseline WHtR after adjusting for potential confounders, including age, sex, blood pressure, total cholesterol, and HbA1c. Statistical significance was defined as a P<0.05.
The baseline characteristics of the participants are presented in Table 1. The mean age was 41 years. The mean WHtR was 0.50 and the mean HbA1c was 5.68%. The proportion of the participants who had CAC at baseline was 20.5%, and 26.5% of the participants had CAC progression after 4 years.
When the study parameters were compared between those with and without CAC progression, the subjects who had CAC progression were 3 years older than those without CAC progression (Table 2). The subjects who had CAC progression were more obese than those who did not. The baseline fasting blood glucose level was higher in the subjects who had CAC progression compared to those who did not have CAC progression. A larger proportion of subjects in the CAC progression group smoked, although the difference was statistically insignificant. All the lipid profiles were worse in the CAC progression group compared to those without CAC progression. The mean WHtR was higher in subjects with CAC progression (0.51 vs. 0.50, P<0.01).
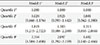
When the subjects were divided into four groups according to baseline WHtR quartiles, the proportion of subjects who had CAC progression after 4 years significantly increased as the WHtR quartiles increased from the 1st to 4th quartiles (18.3%, 18.7%, 28.8%, and 34.2%, P<0.01 for the linear trend) (Fig. 2).
When the AUC for the prediction of CAC progression was calculated with ROC curve analyses among WHtR, BMI, and WC, WHtR was the highest among the three obesity parameters (0.605 vs. 0.596 for BMI, 0.578 for WC).
When the ORs for CAC progression after 4 years were analyzed according to baseline WHtR quartile groups after adjustment for confounding variables, the subjects in the 4th quartile of WHtR showed the highest OR for CAC progression, of 1.602, even after adjustment for confounding variables, such as age, smoking, blood pressure, total cholesterol level and HbA1c (95% confidence interval, 1.040 to 2.466) (Table 3).
This study is the first analysis in the literature of the association of CAC progression with WHtR in apparently healthy Korean men over 4 years of follow-up. A higher proportion of the subjects with high baseline WHtR had CAC progression over 4 years. In addition, the subjects with CAC progression had higher mean baseline WHtR compared to those without CAC progression. Among the three obesity parameters WHtR, BMI, and WC, WHtR showed the highest AUC for the prediction of CAC progression. In logistic regression analysis, the subjects in the highest quartile of baseline WHtR had significantly increased OR for CAC progression over 4 years compared to those in the lowest WHtR quartile. This is the first study in the literature that analyzed the association of WHtR with CAC progression.
Among the various indices of adiposity, the most widely recognized index is BMI, which was first used by the World Health Organization [19]. However, BMI is limited in that even though it is correlated with total body fat, it does not reflect body fat distribution. Moreover, BMI cannot distinguish between a person with excess fat and a person with high muscle mass; therefore, they have the same cardiovascular risk based on BMI alone [20]. Indices such as WC and WHR, which reflect central obesity, have gained popularity due to the limitations of BMI for assessing relative visceral fat distribution [521]. In a study performed in Koreans, the Healthy Twin Study, WC, WHtR, and BMI showed better predictability for metabolic risks over direct body fat measures [22]. In addition, several studies have reported that individuals with the same WC but different heights are unlikely to have the same cardiometabolic risks [23]. Several researchers independently proposed the WHtR as another candidate for the accurate index for the detection of central obesity, correcting the WC for the height of the individual [2425262728]. WHtR correlated well with abdominal fat measured with imaging techniques [2729]. As WHtR is a corrected value within an individual, it has an advantage of the possibility that a single cutoff value could be used for screening in different ethnic groups, while WC requires population-specific boundary values [193031].
In this study, we determined that subjects with a high baseline WHtR had a significantly increased risk for CAC progression over 4 years of follow-up. In addition, the subjects who had CAC progression had significantly higher mean baseline WHtR values compared to those who did not (0.51 vs. 0.50). There are several previously published studies of the association of WHtR with subclinical atherosclerosis. In a cross-sectional study performed in 305 individuals, WHtR and WC correlated better than BMI with arterial stiffness measured by pulsed wave velocity and with subclinical atherosclerosis measured by carotid intima-media thickness (C-IMT) [32]. In another cross-sectional study performed in 562 middle-aged participants in rural Bangladesh, WHR and WHtR appeared to be better predictors of early atherosclerosis assessed by C-IMT than other surrogates of adiposity [33]. In a systemic review that analyzed the role of WHtR as a screening tool for the prediction of CVD and diabetes, WHtR and WC were significant predictors for CVD and diabetes more often than BMI [5]. That study also suggested that a WHtR cutoff of 0.5 could be a suitable global boundary value in both men and women. Although many studies have analyzed the association of baseline WHtR with future CVD events in a prospective manner, the role of WHtR as a predictor for the progression of subclinical atherosclerosis assessed by CAC not determined in a cross-sectional study has not previously been published. Our study is the first that reports the possibility of WHtR as a predictor for CAC progression in a longitudinal analysis.
Our study has various limitations. First, we only analyzed the association between WHtR and CAC progression, but not CVD events. Therefore, we cannot establish any definite cause-effect relationship from the results of our study. Second, we defined CAC progression as any increase in the absolute CACS over 4 years, as previously described in other studies [1718]. Previous studies of CAC progression used various methods to minimize the interscan variability, such as the calculation of percentage change or square root transformation of CACS (SQRT method) [3435]. However, there is no current standardization of how progression should be assessed and exactly what meaningful "progression" constitutes [36]. More studies should be performed to define the optimal method to assess CAC progression. Third, more specific data on personal history of medications, such as lipid-lowering agents or anti-platelet agents, were not available. However, there is no evidence that CAC progression could be prevented or reversed with a single therapeutic intervention, including statins [3738]. Furthermore, there could be limitations in the presumption of WHtR as the correct method to accurately reflect central adiposity. Despite these limitations, as the population of our study was composed of relatively young and apparently healthy subjects from a health screening program, the results of our study could be meaningful in that WHtR could predict CAC progression even in a low risk population. In addition, our study has strength as the first study performed regarding the role of WHtR as the predictor for progression of subclinical atherosclerosis assessed by CACS in apparently healthy Korean men.
In conclusion, male subjects with high baseline WHtR had a higher risk for CAC progression over 4 years in this retrospective study. In addition, the men who had CAC progression had higher mean baseline WHtR compared to those without CAC progression, suggesting that WHtR could be a surrogate marker for the prediction of progression of subclinical atherosclerosis in this relatively young Korean male population without underlying CVD. Further studies are warranted in different ethnic and age groups to clarify the precise role of WHtR as a global adiposity index that has significant predictive power for metabolic diseases and CVD.
Figures and Tables
Fig. 2
Comparison of the proportion of subjects with coronary artery calcium score (CACS) progression in groups divided according to quartile groups of baseline waist-to-height ratio. ANOVA, analysis of variance.

Table 1
Baseline characteristics of the participants (n=1,048)

Table 2
Comparison of the baseline parameters between the groups with and without coronary artery calcification progression after 4 years (n=1,048)

Table 3
Odds ratio for progression of coronary artery calcification according to quartiles of baseline waist-height ratio in 4 years of follow-up
References
1. Hu D, Xie J, Fu P, Zhou J, Yu D, Whelton PK, He J, Gu D. Central rather than overall obesity is related to diabetes in the Chinese population: the InterASIA study. Obesity (Silver Spring). 2007; 15:2809–2816.
2. Balkau B, Deanfield JE, Despres JP, Bassand JP, Fox KA, Smith SC Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007; 116:1942–1951.
3. Lam BC, Koh GC, Chen C, Wong MT, Fallows SJ. Comparison of body mass index (BMI), body adiposity index (BAI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One. 2015; 10:e0122985.
4. Despres JP, Lemieux I, Prud'homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001; 322:716–720.
5. Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010; 23:247–269.
6. Bielak LF, Rumberger JA, Sheedy PF 2nd, Schwartz RS, Peyser PA. Probabilistic model for prediction of angiographically defined obstructive coronary artery disease using electron beam computed tomography calcium score strata. Circulation. 2000; 102:380–385.
7. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995; 92:2157–2162.
8. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008; 358:1336–1345.
9. Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Gronemeyer D, Seibel R, Kalsch H, Brocker-Preuss M, Mann K, Siegrist J, Jockel KH. Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010; 56:1397–1406.
10. Yu JH, Yim SH, Yu SH, Lee JY, Kim JD, Seo MH, Jeon WS, Park SE, Park CY, Lee WY, Oh KW, Park SW, Rhee EJ. The relationship of body composition and coronary artery calcification in apparently healthy Korean adults. Endocrinol Metab (Seoul). 2013; 28:33–40.
11. Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009; 169:186–194.
12. Okwuosa TM, Greenland P, Ning H, Liu K, Bild DE, Burke GL, Eng J, Lloyd-Jones DM. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol. 2011; 57:1838–1845.
13. Okwuosa TM, Greenland P, Ning H, Liu K, Lloyd-Jones DM. Yield of screening for coronary artery calcium in early middle-age adults based on the 10-year Framingham risk score: the CARDIA study. JACC Cardiovasc Imaging. 2012; 5:923–930.
14. National Glycohemoglobin Standardization Program: List of NGSP certified methods. cited 2016 Jan 20. Available from: http://www.ngsp.org/docs/methods.pdf.
15. Schwartz KL, Monsur JC, Bartoces MG, West PA, Neale AV. Correlation of same-visit HbA1c test with laboratory-based measurements: a MetroNet study. BMC Fam Pract. 2005; 6:28.
16. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999; 74:243–252.
17. O'Neal WT, Efird JT, Qureshi WT, Yeboah J, Alonso A, Heckbert SR, Nazarian S, Soliman EZ. Coronary artery calcium progression and atrial fibrillation: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2015; 8:e003786.
18. Koulaouzidis G, Charisopoulou D, Maffrett S, Tighe M, Jenkins PJ, McArthur T. Coronary artery calcification progression in asymptomatic individuals with initial score of zero. Angiology. 2013; 64:494–497.
19. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva: WHO;1998.
20. Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004; 363:163.
21. Xu Z, Qi X, Dahl AK, Xu W. Waist-to-height ratio is the best indicator for undiagnosed type 2 diabetes. Diabet Med. 2013; 30:e201–e207.
22. Lee K, Song YM, Sung J. Which obesity indicators are better predictors of metabolic risk?: healthy twin study. Obesity (Silver Spring). 2008; 16:834–840.
23. Hsieh SD, Yoshinaga H. Do people with similar waist circumference share similar health risks irrespective of height? Tohoku J Exp Med. 1999; 188:55–60.
24. Hsieh SD, Yoshinaga H. Is there any difference in coronary heart disease risk factors and prevalence of fatty liver in subjects with normal body mass index having different physiques? Tohoku J Exp Med. 1995; 177:223–231.
25. Hsieh SD, Yoshinaga H. Waist/height ratio as a simple and useful predictor of coronary heart disease risk factors in women. Intern Med. 1995; 34:1147–1152.
26. Hsieh SD, Yoshinaga H. Abdominal fat distribution and coronary heart disease risk factors in men-waist/height ratio as a simple and useful predictor. Int J Obes Relat Metab Disord. 1995; 19:585–589.
27. Ashwell M, Lejeune S, McPherson K. Ratio of waist circumference to height may be better indicator of need for weight management. BMJ. 1996; 312:377.
28. Lee JS, Aoki K, Kawakubo K, Gunji A. A study on indices of body fat distribution for screening for obesity. Sangyo Eiseigaku Zasshi. 1995; 37:9–18.
29. Soto Gonzalez A, Bellido D, Buno MM, Pertega S, De Luis D, Martinez-Olmos M, Vidal O. Predictors of the metabolic syndrome and correlation with computed axial tomography. Nutrition. 2007; 23:36–45.
30. Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005; 56:303–307.
31. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, Kim EM, Suh SH, Shin HJ, Lee KR, Lee KY, Lee SY, Lee SY, Lee SK, Lee CB, Chung S, Jeong IK, Hur KY, Kim SS, Woo JT. Committee of Clinical Practice Guidelines. Korean Society for the Study of Obesity. 2014 Clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014; 29:405–409.
32. Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, Garcia-Ortiz L. Vasorisk group. Abdominal obesity vs general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc Disord. 2012; 12:3.
33. Ge W, Parvez F, Wu F, Islam T, Ahmed A, Shaheen I, Sarwar G, Demmer RT, Desvarieux M, Ahsan H, Chen Y. Association between anthropometric measures of obesity and subclinical atherosclerosis in Bangladesh. Atherosclerosis. 2014; 232:234–241.
34. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010; 3:1229–1236.
35. Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004; 182:1327–1332.
36. McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010; 56:1613–1622.
37. Houslay ES, Cowell SJ, Prescott RJ, Reid J, Burton J, Northridge DB, Boon NA, Newby DE. Scottish Aortic Stenosis and Lipid Lowering Therapy, Impact on Regression trial Investigators. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart. 2006; 92:1207–1212.
38. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005; 46:166–172.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download