Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
ROC Curve of electrocardiographic parameters that distinguished normal coronary from non-critical and significant stenosis groups. QTmax: maximum QT interval, QTd: QT dispersion, QTcmax: corrected QT maximum interval, QTcd: corrected QT dispersion, QTdR: QT dispersion ratio, Pmax: maximum p wave interval, PWD: P wave dispersion.

Fig. 2
ROC Curve of electrocardiographic parameters that distinguished coronary arteries with <50% narrowing from the ones with ≥50% narrowing. QTc: corrected QT, QTmax: maximum QT interval, QTcmax: corrected QT maximum interval, QTcd: corrected QT dispersion, QTdR: QT dispersion ratio, Pmax: maximum p wave interval, PWD: P wave dispersion.
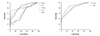
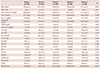
Table 1
Categorical distribution of the socio-demographic data of patients

Table 2
Distribution of the groups according to the laboratory and clinical characteristics
*All of the p values were <0.001 on comparison of Gensini score among the groups. FBG: fasting blood glucose, HbA1c: glycated hemoglobin A1c, LDL-C: low density lipoprotein cholesterol , HDL-C: high density lipoprotein cholesterol , BUN: blood urea nitrogen, AST: aspartat aminotransferase, ALT: alanine aminotransferase, Na: sodium, K: potassium, Hb: hemoglobin, Hct: hematocrit, MPV: mean platelet volume, LVH: left ventricular hypertrophy, LA: left atrium, RA: right atrium, LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, EF: ejection fraction
Table 3
Distribution of ECG findings among the groups

*All of the p values were <0.05 on comparison of these parameters among the groups. Pmax: maximum p wave interval, Pmin: minimum p wave interval, PWD: P wave dispersion, QTmax: maximum QT interval, QTmin: minimum QT interval, QTd: QT dispersion, QTcmax: corrected QT maximum interval, QTcmin: corrected QTc minimum interval, QTcd: corrected QT dispersion, QTdR: QT dispersion ratio
Table 4
Correlation of ECG findings of the patients with Gensini scores

| Gensini score | ||
|---|---|---|
| r | p | |
| QTmax | 0.239 | <0.001 |
| QTmin | 0.015 | 0.062 |
| QTd | 0.421 | <0.001 |
| QTcmax | 0.263 | <0.001 |
| QTcmin | -0.005 | 0.059 |
| QTcd | 0.387 | <0.001 |
| QTdR | 0.467 | <0.001 |
| Pmax | 0.582 | <0.001 |
| Pmin | 0.116 | 0.112 |
| PWD | 0.656 | <0.001 |
ECG: electrocardiography, QTmax: maximum QT interval, QTmin: minimum QT interval, QTd: QT dispersion, QTcmax: corrected QT maximum interval, QTcmin: corrected QTc minimum interval, QTcd: corrected QT dispersion, QTdR: QT dispersion ratio, Pmax: maximum p wave interval, Pmin: minimum p wave interval, PWD: P wave dispersion
Table 5
Cut-off, sensitivity, specificity, AUC, and p values of ECG parameters that distinguish Group 1 and Group 2 from Groups 3, 4 and 5

Table 6
Cut-off, sensitivity, specificity, AUC, and p of ECG parameters that distinguish a coronary artery with <50% narrowing from a coronary artery with ≥50% narrowing





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download