Abstract
Background and Objectives
Apurinic/apyrimidinic endonuclease 1/redox effector factor-1 (APE1/Ref-1) is a multifunctional protein involved in the DNA base excision repair pathway, inflammation, angiogenesis, and survival pathways. We investigated serum APE1/Ref-1 in patients with coronary artery disease (CAD).
Subjects and Methods
Serum APE1/Ref-1 was measured with a sandwich enzyme-linked immunosorbent assay from 360 patients who received coronary angiograms. They were divided into two groups; a control (n=57) and a CAD group (n=303), the latter included angina (n=128) and myocardial infarction (MI, n=175).
Results
The levels of APE1/Ref-1 were higher in the CAD than the control (0.63±0.07 vs. 0.12±0.07 ng/100 µL, respectively; p<0.01). They were also higher in MI than angina (0.81±0.10 vs. 0.38±0.11 ng/100 µL, respectively; p<0.01) and different according to the thrombolysis in myocardial infarction (TIMI) flow (0.88±0.09 for TIMI flow 0, 1, 2 vs. 0.45±0.13 ng/100 µL for TIMI flow 3, p<0.01) in acute coronary syndrome. In correlation analysis, the levels of APE1/Ref-1 were positively correlated with Troponin I (r=0.222; p<0.0001) and N-terminal pro-B type natriuretic peptide (NT-proBNP, r=0.217; p<0.0001) but not high sensitivity to C-reactive protein. Also, they revealed a negative correlation with ejection fraction (EF, r=-0.221; p=0.002). However, there were no significant differences among the three groups, were divided by their levels of APE1/Ref-1, for major adverse cardiovascular events (death, recurrent MI, stroke, revascularization) (8.2 vs. 14.0 vs. 12.5%, p=ns).
Coronary artery disease (CAD) is increasing significantly. Although various treatments, such as medication, intervention, and surgery are widely used to improve survival rates, CAD is still a leading cause of morbidity and mortality worldwide.1)2) Early detection and prognosis evaluation of CAD are very important issues. Biomarkers are one tool to better identify high risk individuals, to accurately diagnose disease conditions, and to effectively prognosticate and treat patients with thedisease.1)2)
Apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1) is a multifunctional protein that is mainly located in the nucleus. APE1 is involved in the base excision repair pathway, and Ref-1 acts as a reductive activator of many transcription factors in controlling different cellular process such as apoptosis, inflammation, proliferation, angiogenesis, and is involved in survival pathways.3)4) There are many reports that alterations in the expression, subcellular localization, and activities of APE1/Ref-1 are associated with various human diseases, including cancer, neurodegenerative disease, and hypertension.4)5)6) Because DNA damage plays an important role in the pathogenesis of CAD,7)8)9) alterations in APE1/Ref-1 can occur in CAD. However, there are no studies about the relationship between serum APE1/Ref-1 concentration and CAD. The purpose of this study was to verify the potential of APE1/Ref-1 as a biomarker for the early diagnosis and prognostic evaluation of serum APE1/Ref-1 in CAD by investigating serum APE1/Ref-1 in patients with and without CAD.
This study was approved by the Chungnam National University Hospital Institutional Review Board. We enrolled patients who underwent coronary angiograms (CAG) at the department of cardiology of the Chungnam National University and had signed informed consent forms between January 2012 and May 2013. The patients were classified as controls, consisting of individuals with no significant coronary artery stenosis, or patients with coronary artery disease. The CAD group, with chest pain caused by significant coronary artery stenosis, included stable, unstable angina, and acute myocardial infarction (AMI; Non-ST elevation myocardial infarction, NSTEMI and ST elevation myocardial infarction, STEMI). STEMI was diagnosed when patients came to the hospital with chest pain lasting for more than 30 minutes within the past 12 hours and showing an ST segment elevation of ≥1 mm in more than two adjacent limb leads or ≥2 mm in more than two contiguous precordial leads. NSTEMI was diagnosed in patients with an elevation of cardiac biomarkers and chest pain lasting more than 10 minutes and without evidence of ST segment elevation. CAG in NSTEMI was conducted within 72 hours of admission. Exclusion criteria included controls who had prior CAD or any infectious disorders that caused chest pain, such as pneumonia, cholecystitis, or gastrointestinal disorders. Also, patients with cancer or neurodegenerative diseases such as Alzheimer's disease or Parkinson's disease (known to be associated with an elevated APE1/Ref-1) were excluded from both the control and CAD groups.
All blood samples were taken into vacuum tubes before the CAG. Serum was separated by centrifugation at 3000 rpm for 10 minutes at room temperature and then re-centrifuged at 5000 rpm for 5 minutes to obtain cell-free serum, which was stored in liquid nitrogen until use. A sandwich enzyme-linked immunosorbent assay (ELISA) was used to quantify serum APE1/Ref-1 levels. Briefly, 96-microwell plates (Nunc) were pre-coated overnight with 100 µL of a 1:1000 dilution of a rabbit anti-APE1/Ref-1 antibody (Abcam, Cambridge, UK) in coating buffer (0.5 M carbonate buffer, pH 9.6) in each well. After blocking with a blocking buffer {5% bovine serum albumin in phosphate buffered saline containing 0.05% Tween 20, (PBS-T)} at room temperature for 60 minutes, 100 µL of sample was added to the wells and the plates were incubated at 4℃ for 90 minutes, and then washed 5 times with PBS-T. This was followed by the addition of 100 µL of a 1:1000 dilution of mouse anti-APE1/Ref-1 antibody (Abcam, Cambridge, UK), and further incubation at room temperature for 2 hours. The plate was then washed 7 times with PBS-T, and 100 µL of horseradish peroxidase-conjugated secondary antibody (1:5000) was added, followed by incubation at room temperature for 30 minutes. After further washing, 100 µL of freshly prepared tetramethylbenzidine substrate was added to the wells. The color development reaction was stopped by adding 100 µL of 2.5 M H2SO4, and the absorbance was measured at 450 nm using an automatic microtiter plate reader (Sunrise Xfluor4; Tecan Systems, Inc., San Jose, CA, USA). Each sample was assayed in duplicate, and mean values were determined. Purified recombinant human APE1/Ref-1 (1 mg/mL) was serially diluted (5-fold) and used in a concentration series from 0.16-20 ng/100 µL to establish a standard curve.
Basic demographic data, past clinical history, and blood chemistry test results were collected from the patients' medical records. N-terminal pro-B type natriuretic peptide (NT pro-BNP), high sensitivity C-reactive protein (hsCRP), creatinine kinase (CK), CK-MB, and troponin-I were analyzed together.
Patients were monitored for an additional 24.0±7.4 months to determine the occurrence of major adverse clinical events (MACEs). MACEs included death, recurrence of myocardial infarction (MI), stroke, and revascularization including new vessel revascularization, target vessel revascularization, and target lesion revascularization. The revascularization that was planned at the time of enrollment was excluded, and it was only included when it was performed due to the progression of symptoms or stenosis. All events were determined by reviewing the patients' medical records with regular clinical follow-up. In patients without regular clinical follow-up, telephone interviews were conducted and deaths were checked from the nationwide database from the National Health Institute Service in Korea.
The data were analyzed using standard software (SPSS version 20.0, IBM Co., Chicago, IL, USA). Categorical variables are presented as frequency and percentage. Summary data were expressed as mean±standard error of the mean or percentage of patients. Categorical variables were compared with χ2 test and continuous variables were compared with Student's t-test or analysis of variance. Bivariate correlation analysis was used to compare APE1/Ref-1 with other cardiovascular biomarkers. Cox proportional hazard regression was used to compute the impact of adverse clinical events at each time point. A p value less than 0.05 was considered statistically significant.
We enrolled 360 patients in total (237 men; mean age 64±12 years old). The patients included 57 controls (15.8%) and 303 CAD patients (84.2%), including angina (n=128, 35.6%; stable angina, n=52, 14.4%; unstable angina, n=76, 21.1%) and MI (n=175, 48.6%; NSTEMI, n=55, 15.3%; STEMI, n=120, 33.3%). The patients' clinical characteristics are presented in Table 1. After CAG, percutaneous coronary intervention was performed at the physician's discretion.
The serum levels of APE1/Ref-1 in patients with CAD were significantly elevated compared to those of the control group (0.63±0.07 for the CAD group vs. 0.12±0.07 ng/100 µL for the control group, p<0.01) (Fig. 1A). There were no gender differences (0.59 ±0.08 ng/100 µL for men vs. 0.47±0.10 ng/100 µL for women). The same results were observed in both groups (control vs. CAD; 0.19±0.13 ng/100 µL vs. 0.64±0.89 ng/100 µL for men, 0.07 ±0.06 ng/100 µL vs. 0.60±0.13 ng/100 µL for women, p<0.02). The receiver operating characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of APE1/Ref-1 in detecting CAD. The ROC curve from serum samples of 57 controls and 303 patients with CAD resulted in an area under the curve (AUC) of 0.66 (95% confidence interval, 0.592 to 0.729) (Fig. 1B). The sensitivity and specificity of APE1/Ref-1 were estimated at the optimal cut-off value in order to maximize sensitivity, specificity, and likelihood ratio. On this basis, the optimal combination of sensitivity and specificity were determined to be 0.36 and 0.95, using a cut-off value of 0.16 ng/100 µL.
The levels of APE1/Ref-1 were higher in patients with MI than angina in the CAD group (0.81±0.101.30 for MI vs. 0.38±0.11 ng/100 µL for angina, p<0.01) (Fig. 2A). There were not significantly different between NSTEMI and STEMI (0.64±0.12 for NSTEMI vs. 0.89±0.13 ng/100 µL for STEMI, p>0.05). There were significantly higher in STEMI than in the control group, but not in NSTEMI (Fig. 2B). Also, we analyzed the levels of serum APE1/Ref-1 according to the thrombolysis in myocardial infarction (TIMI) grade flow in patients with acute coronary syndrome (ACS), including unstable angina, NSTEMI, and STEMI. The lower TIMI flow was significantly correlated with higher APE1/Ref-1 (0.12±0.07 for control vs. 0.45±0.13 for TIMI flow 3 vs. 0.88±0.09 ng/100 µL for TIMI flow 0-2, p<0.01) (Fig. 2C). Four days after the CAG, serum APE1/Ref-1 was re-measured from 37 patients who were selected randomly among the STEMI patients. The levels of the biomarker declined from 1.62±0.27 ng/100 µL to 0.35±0.23 ng/100 µL (Fig. 2D).
We compared the serum APE1/Ref-1 with NT-proBNP, troponin I, and hsCRP which have all been used as cardiovascular biomarkers. In correlation analysis, NT-proBNP and troponin I at admission were positively correlated with serum APE1/Ref-1 (r=0.217, p<0.01 for NT pro-BNP, and r=0.222, p<0.01 for troponin I) (Fig. 3A and B). There was no significant correlation with hsCRP (Fig. 3C). The ejection fraction, which is known to be associated with prognosis, was negatively correlated with serum APE1/Ref-1 (r=-0.221, p<0.01) (Fig. 3D). Neither peak troponin I nor peak CK-MB had a significant correlation.
No MACEs were observed in the control group during the follow-up period. There were 35 MACEs in the CAD group during the follow-up of 24.0±7.4 months, including 26 deaths, 1 stroke, and 8 revascularizations. The incidence was not different between the angina and MI groups (total MACE 9.4% for angina vs. 13.1% for MI; p>0.05) (Table 2). We compared MACEs among three groups divided by serum APE1/Ref-1 concentrations; <0.10, >0.10 and <1.10, and >1.10. There were no significant differences between the three groups (Table 2). Cox proportional hazard regression also showed that serum APE1/Ref-1 was not associated with MACE. Only hsCRP appeared to be associated with MACE (hazard ratio 1.029, p<0.01), but not NT-proBNP or ejection fraction.
In this report, we demonstrate, for the first time, that serum APE1/Ref-1 is increased in patients with coronary artery disease, and that it is correlated with NT pro-BNP, troponin I and ejection fraction. The cut-off value of APE1/Ref1 to distinguish the CAD group from the control group is 0.16 ng/100 µL, providing the optimal combination of sensitivity, specificity, and likelihood ratio.
There are many conditions which reveal elevated levels of APE-1/Ref-1, such as cancer, inflammatory state, hypertension, carotid atherosclerosis, and other undescribed conditions.3)4) In relation to cardiovascular disease, Jeon et al.10) reported that the mean arterial blood pressure in APE1/Ref-1 heterozygotic mice is significantly higher than in wild type mice.10)11)12) Naganuma et al.5) suggested that the APE1/Ref-1 gene appears to be a susceptibility gene for essential hypertension. Martinet et al.9) reported that APE1/Ref-1 as a DNA repair enzyme is elevated in human carotid atherosclerotic plaques. In other words, APE1/Ref-1 is increased and activated for radiation, reactive oxygen species (ROS), ischemia/reperfusion (IR), hypoxia and other conditions. Although many reports have showed that APE1/Ref-1 is localized to the nucleus and the underlying mechanism for serum APE1/Ref-1 existence is completely elucidated, it was recently reported that APE1/Ref-1 was secreted into the bloodstream in response to lipopolysaccharides.13)14) In lung and bladder cancer, elevation of serum APE1/Ref-1 has been identified.15)16) Considering that CAD is associated with chronic inflammation, ROS, and IR injury, the elevation of APE1/Ref-1 in the sera of patients with CAD may be natural and expected. The increased inflammatory response from plaque rupture in acute coronary syndrome, especially in myocardial infarctions, may explain the higher levels of serum APE1/Ref-1 in MI than in angina, as shown in the present study. However, the results show that the AUC (0.66) is too low and the sensitivity (0.36) make the APE-1/Ref-1 level would not be a good candidate as a cardiac specific biomarker in the diagnosis of CAD. It might be related other possible conditions which affect the levels of APE1/Ref-1, such as inflammation, cancer, or other atherosclerosis or ischemic disease.3)4) Also, there are overlapping groups between the APE1/Ref-1 levels in the control and CAD groups. The patients in the control group who had hypertension or diabetes showed relatively higher levels of APE1/Ref-1. The patients with NSTEMI show wide range of APE1/Ref-1 concentrations from 0 to 2.97 ng/100 µL. The wide range of APE1/Ref-1 might be related to the heterogeneity of patients with NSTEMI, such as hypertension, diabetes, smoking, and the various time between the disease onset and the blood collection (in this data, APE1/Ref-1 decreased after a few days). We did not analyze the relationship between APE1/Ref-1 and the NSTEMI risk score. We think the overlapping itself shows that APE1/Ref-1 would not be the best biomarker. The level of low density lipoprotein cholesterol, a biomarker of atherosclerosis, shows significant overlap between the control and CAD groups. Though our data showed no significant differences between the control and NSTEMI groups, if the cuff-off value is increased, differences will be evident. Even though serum APE1/Ref-1 shows a limited diagnostic power, and ability differentiate acute coronary syndrome in those who present with chest pain in the emergency room, our data demonstrated that the serum levels of APE1/Ref-1 in patients with CAD were significantly elevated compared to those of the control group. A larger study is needed to verify the usefulness of serum APE1/Ref-1 as a diagnostic cardiac biomarker.
What is the role of elevated APE1/Ref-1 in CAD? Is it a just inflammation marker? Our data shows that APE1/Ref 1 has no correlation with hsCRP. This may mean that the elevation of serum APE1/Ref-1 in CAD is not a non-specific result solely due to inflammation. It is commonly accepted that APE1/Ref-1 activation is required for protecting cells from oxidative injuries.17)18)19) Moreover, it has been demonstrated that the overexpression of APE1/Ref-1 is able to prevent H2O2-induced cell death in different cells systems.20)21) Recombinant APE1/Ref-1 protein treatment inhibited tumor necrosis factor-α-induced vascular cell adhesion molecule expression in cultured endothelial cells.13) So, we suggest that the elevation of APE1/Ref-1 is related to oxidative injury of the myocardium and vessel wall, and the reason for the elevation is to prevent cell death. The serum levels of APE1/Ref-1 were not correlated with the severity of myocardial injury, such as peak troponin I or peak CK-MB. Even though serum APE1/Ref-1 positively correlated with NT-proBNP and troponin I, and was negatively correlated with ejection fraction, we suggest that serum APE1/Ref-1 elevation might be related to vessel well damage not myocardial damage. We were unable to identify the usefulness of APE1/Ref-1 as a prognostic biomarker because it was not correlated with clinical outcome. Although serum APE1/Ref-1 was more elevated in patients with MI, it did not show any association with adverse clinical events. These results might be related to the small sample size. In this study, there were only 35 MACEs in 354 cases during a follow-up period of 24.0±7.4 months. Any factors affecting adverse clinical outcomes, such as NT-proBNP, high troponin I, and ejection fraction, with the exception of hsCRP, were not associated with MACEs. That means the sample size of this study was too small to confirm these effects. Also, the fact that MI patients have a worse prognosis compared with angina was not seen in the present study. So, we think that a larger study is needed to evaluate the relationship between serum APE1/Ref-1 and clinical outcomes and to determine its usefulness for prognosis.
Our study has several limitations related to its small sample size. Although we included blood samples from all groups we examined, all measurements of serum APE1/Ref-1 were not taken at the same time and within six months after the initial collection. Even though we excluded diseases such as cancer, Alzheimer's disease and Parkinson's disease, we could not completely eliminate other possible conditions that could cause changes in APE1/Ref-1. The possibility that medication could be responsible for alterations of APE1/Ref-1 should be considered, even though we followed the protocol for drug therapies.
In conclusion, we found that serum APE1/Ref-1 may be increased in patients with CAD. It is correlated with other cardiovascular biomarkers such as troponin I, N-terminal pro-B type and ejection fraction. However, we need larger studies to determine its usefulness as a novel biomarker for the early diagnosis and prognostic prediction in CAD.
Figures and Tables
Fig. 1
Serum APE1/Ref-1 in the control and coronary artery disease (CAD) groups. A. Serum APE1/Ref-1 was assayed by an enzyme-linked immunosorbent assay in 57 controls and 303 patients with CAD. The results are presented as a scatter plot (p<0.01 vs. control). Serum APE1/Ref-1 is elevated in CAD. B. Receiver operating curves of serum APE1/Ref-1 for CAD. The area under curve (AUC) by APE1/Ref-1 is 0.66. APE1/Ref-1: apurinic/apyrimidinic endonuclease 1/redox effector factor 1.
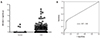
Fig. 2
Serum APE1/Ref-1 in coronary artery disease. Serum APE1/Ref-1 was assayed by ELISA. Each bar shows the mean±standard error of the mean. A. The levels of serum APE1/Ref-1 are higher in patients with myocardial infarction (MI) than angina. *p<0.01, control vs. angina and angina vs. MI, **p<0.001, control vs. MI. B. Serum APE1/Ref-1 is not significantly different between the non-ST elevation myocardial infarction (NSTEMI) and STEMI patients. *p<0.01, ns; non-specific. C. Serum APE1/Ref-1 levels are associated with thrombolysis in myocardial infarction (TIMI) grade flow in acute coronary syndrome (ACS). TIMI flow 0-2 shows higher levels of APE1/Ref-1 than TIMI 3. *p<0.01, TIMI 3 vs. TIMI 0-2. D. The elevated APE1/Ref-1 decreased 4 days after CAG from 1.62±0.27 ng/100 µL to 0.35±0.23 ng/100 µL. *p<0.01. APE1/Ref-1: apurinic/apyrimidinic endonuclease 1/redox effector factor 1, CAG: coronary angiograms.
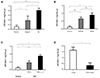
Fig. 3
Serum APE1/Ref-1 and other cardiovascular biomarkers. A-B. Serum APE1/Ref-1 is positively correlated with N-terminal pro-B type natriuretic peptide (NT-proBNP) and troponin I (r=0.217, p<0.01 for NT-proBNP and r=0.222, p<0.01 for troponin I). C. The high sensitivity C-reactive protein (hsCRP) does not show any significant correlation with APE1/Ref-1. D. Ejection fraction (EF), which is measured by Simpson's method on transthoracic echocardiography, has a negative correlation (r=-0.221, p<0.01). APE1/Ref-1: apurinic/apyrimidinic endonuclease 1/redox effector factor 1.

Table 1
Baseline characteristics (n=360)
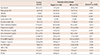
Data are expressed as means±standard deviation. MI: myocardial infarction, CAD: coronary artery disease, DM: diabetes mellitus, HTN: hypertension, HDL-cholesterol: high density lipoprotein-cholesterol, LDL-cholesterol: low density lipoprotein-cholesterol, NT pro-BNP: N-terminal pro-B type natriuretic peptide, Hs-CRP: high sensitive C-reactive protein, HbA1c: hemoglobin A1C, Hb: hemoglobin, EF: ejection fraction
Table 2
Serum APE1/Ref-1 and clinical outcomes

Acknowledgements
This study was supported by grants from the Korean Society of Cardiology 2012, Chungnam National University Hospital Research Fund 2012, and Basic Science Research Program through the National Research Foundation of Korea (NRF), fund by the Ministry of Education (NRF-2014R1A6A1029617). The biospecimens used in this study were provided by the Biobank of Chungnam University Hospital, a member of Korea Biobank.
References
1. Kim HC. Clinical utility of novel biomarkers in the prediction of coronary heart disease. Korean Circ J. 2012; 42:223–228.
2. Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006; 113:2335–2362.
3. Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009; 11:601–620.
4. Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK. APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med. 2014; 46:e106.
5. Naganuma T, Nakayama T, Sato N, et al. Haplotype-based case-control study on human apurinic/apyrimidinic endonuclease 1/redox effector factor-1 gene and essential hypertension. Am J Hypertens. 2010; 23:186–191.
6. Zhang Y, Wang J, Wang D, Xin X. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) in human ovarian cancer and indentification of the therapeutic potential of APE1/Ref-1 inhibitor. Int J Oncol. 2009; 35:1069–1079.
7. Botto N, Rizza A, Colombo MG, et al. Evidence for DNA damage in patients with coronary artery disease. Mutat Res. 2001; 493:23–30.
8. Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 2006; 71:259–268.
9. Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002; 106:927–932.
10. Jeon BH, Gupta G, Park YC, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004; 95:902–910.
11. Patterson C. Blood pressure control goes nuclear. Circ Res. 2004; 95:849–851.
12. Song SH, Cho EJ, Park MS, et al. Redox regulating protein APE1/Ref-1 expression is increased in abdominal aortic coarctation-induced hypertension rats. J Korean Soc Hypertens. 2012; 18:126–135.
13. Park MS, Lee YR, Choi S, et al. Identification of plasma APE1/Ref-1 in lipopolysaccharide-induced endotoxemic rats: implication of serological biomarker for an endotoxemia. Biochem Biophys Res Commun. 2013; 435:621–626.
14. Choi S, Lee YR, Park MS, et al. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem Biophys Res Commun. 2013; 435:403–407.
15. Dai N, Cao XJ, Li MX, et al. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS One. 2013; 8:e58001.
16. Shin JH, Choi S, Lee YR, et al. APE1/Ref-1 as a serological biomarker for the detection of bladder cancer. Cancer Res Treat. 2015; 01. 02. DOI: 10.4143/crt.2014.074. [Epub].
17. Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005; 7:367–384.
18. Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000; 461:83–108.
19. Pines A, Perrone L, Bivi N, et al. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005; 33:4379–4394.
20. Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst). 2005; 4:367–379.
21. Wang D, Luo M, Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004; 3:679–686.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download