Abstract
Spontaneous coronary artery dissection (SCAD) is an uncommon cause of acute coronary syndrome which may be related to lethal condition. Although several modalities including medical therapy have been suggested, agreement on optimal treatment has not yet been determined. We describe a case of SCAD which was presented as ST-segment elevation myocardial infarction, and treated successfully with medical treatment. Coronary angiography, intravascular ultrasound and multi-detector computed tomography showed the serial changes of this disease entity.
Spontaneous coronary artery dissection (SCAD) is a rare and fatal cause of acute coronary syndrome (ACS) that has been reported by autopsy in many cases historically.1) The clinical diagnosis of SCAD has increased because coronary angiography (CAG) and intravascular ultrasound (IVUS) available for providing detailed morphological information of vessel layers is frequently performed for evaluation of ACS. Although several modalities including percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG) and medical therapy have been performed to manage patients with SCAD, no treatment guideline has yet been established. We report a case of SCAD which was presented as ST-segment elevation myocardial infarction (STEMI) and treated successfully with medical treatment.
A 53 year-old woman was transferred to the emergency department due to chest pain via a district hospital. Her risk factor for coronary artery disease was hypertension on medication. She denied any prior history of pregnancy, cocaine or amphetamine medication, chest trauma, connective tissue disease and family history of premature coronary artery disease.
On arrival, her vital signs were stable except for blood pressure reading of 140/84 mmHg. The physical examination revealed no evidence of tenderness on the anterior chest and no sign of joint swelling or tenderness. Initial electrocardiogram revealed anterior and inferior ST-segment elevations. Initial chest X-ray showed no sign of pulmonary edema. The levels of total cholesterol, low density lipoprotein-cholesterol (LDL-C) and cardiac enzymes were elevated. The patient's total cholesterol, triglyceride, high density lipoprotein-cholesterol, and LDL results were 236 mg/dL (0-200 mg/dL), 98 mg/dL (0-150 mg/dL), 41 mg/dL (35-55 mg/dL), 169 mg/dL (55-155 mg/dL), respectively. Creatine kinase-MB, and troponine-I levels were 113 ng/mL (0-2.8 ng/mL), and 26 ng/mL (0-0.045 ng/mL). Glucose was 95 mg/dL (70-110 mg/dL), and creatinine was 0.81 mg/dL (0.7-1.4 mg/dL). Antinuclear antibody and antinuclear and antineutrophil cytoplasmic autoantibodies were all negative. Echocardiography showed antero-apical wall hypokinesia.
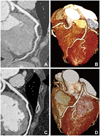
Suspecting myocardial infarction, we performed emergent CAG. In the examination, a long and smooth lesion from the mid to distal left anterior descending artery (LAD) was detected, which showed an abrupt transition from the proximal LAD (Fig. 1A). IVUS confirmed the dissecting flap with intramural hematoma compressing the true lumen at the distal LAD (Fig. 1C), and ectatic dilatation of the proximal LAD (internal diameter: approximately 6 mm). The intimal tear site was not detected on the IVUS examination. During the procedure, the patient did not complain of chest pain and all vital signs were stable. Because the intramural hematoma was located at distal LAD and the Thrombolysis in Myocardial Infarction flow grade was three, we did not conduct any additional interventional treatments for the patient. Multi-detector computed tomography (MDCT) of coronary artery was undertaken three days later using a Siemens 64-slice scanner. We observed a luminal narrowing of the distal LAD with a perivascular low attenuation corresponding to hematoma and ectatic change of the left coronary artery (Fig. 2A and B).
The patient was treated with aspirin, clopidogrel, bisoprolol, valsartan, and diltiazem, and did not suffer from any discomfort after discharge. Follow-up examinations were performed 6 months later. There was no further discrepancy of vessel size on CAG (Fig. 1B). On the follow-up IVUS, the intramural hematoma in distal LAD was not seen (Fig. 1D), and ectatic dilatation of the proximal LAD persisted. Follow-up MDCT of the coronary artery demonstrated dilatation of lumen size at the distal LAD with resolution of perivascular hematoma (Fig. 2C and D).
SCAD is developed when primary or secondary dissection occurs in the coronary artery. It separates coronary arterial layers and formed hematoma between the layers which compress the true lumen of the coronary artery and induce ischemia. The incidence of SCAD is reported from 0.1% up to 1.1% of patients referred for CAG.2) The etiology is not fully known, but atherosclerosis is considered to be the most common causal factor for SCAD.3) Hormonal changes in women who are in peripartum period or taking oral pills, iatrogenic, secondary to chest trauma, connective tissue disease and vasculitis, seem to be related to SCAD.4-6) In cases of SCAD with obscure CAG findings, IVUS can be helpful by detecting the presence of an intramural hematoma in the outer layer of the media compressing the true lumen.5) The detection of intimal tearing flap by IVUS is not always available maybe due to spontaneous clotting at the tearing point.7) In addition to IVUS, according to development of CT spatial resolution, MDCT of the coronary artery can visualize the extent and thickness of hematoma in the assessment of SCAD.5)
The therapeutic strategy is generally determined by the clinical condition and image findings.3)5) If vital conditions are stable and ischemia is not ongoing, SCAD may be treated medically similar to medical treatment of ACS. Thrombolytic therapy in SCAD is not recommended due to the extension of dissection resulting in poor prognosis. PCI is recommended if ongoing myocardial ischemia is persistent. CABG can be considered in cases of dissection of left main coronary artery or multivessel involvement.
Although our patient was presented with STEMI, the initial findings of CAG and IVUS were compatible with SCAD, and vital signs were stable. We were able to avoid invasive procedures like coronary stenting, and short-term follow-up with MDCT resulted in more confidence in the treatment. In addition to follow-up CAG, serial check-up using IVUS and MDCT could clearly assess the resolution of hematoma and the acquisition of the correct true lumen. Therefore, we think that IVUS and MDCT can be useful to diagnose, decide the optimal treatment and identify the progress of disease in the case of SCAD presented with STEMI.
Figures and Tables
Fig. 1
Serial findings of coronary angiography (CAG) and intravascular ultrasound (IVUS) of distal left anterior descending artery (LAD). A: initial CAG showed a long and smooth lesion from the mid to distal LAD which was abruptly transitted from the proximal LAD. B: follow-up CAG revealed improved state of distal LAD. C: in the initial IVUS. The huge extramural hematoma compressing the true lumen of distal LAD. D: follow-up IVUS revealed resolved hematoma of distal LAD.
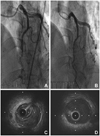
Fig. 2
Serial findings of multi-detector computed tomography (MDCT) of distal left anterior descending artery (LAD). A and B: initial MDCT manifest compressing extramural hematoma and narrowed true lumen of distal LAD. C and D: follow-up MDCT revealed disappeared hematoma and normal luminal diameter of distal LAD.
References
1. DeMaio SJ Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989. 64:471–474.
2. Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry Study. Catheter Cardiovasc Interv. 2009. 74:710–717.
3. Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol. 1998. 87:961–970.
4. Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection): coincidence or pathogenetic significance? Cardiovasc Res. 1997. 33:527–532.
5. Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010. 96:801–808.
6. Park SH, Park HS, Lee JH, et al. A case of coronary artery dissection after aortic replacement in acute type A aortic dissection. Korean Circ J. 2009. 39:428–433.
7. Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002. 89:466–468.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download