Abstract
Background and Objectives
Ventricular assist systems are used in patients with end-stage heart failure to prolong life or as a bridge to transplantation. Several types of ventricular assist systems have been developed and they are now being used. We developed a new Biomedlab® electro-mechanical implantable ventricular assist device (IVAD) and we performed in vivo experimentation to evaluate the durability and safety of the device, as well as its hematologic effect.
Subjects and Methods
We implanted the newly developed IVAD in the pre-peritoneal cavity of 5 Hallstein calves. The inflow tract was inserted through the left ventricular apex, and the outflow tract was anastomosed to the descending thoracic aorta. Postoperatively, we administered heparin intravenously for 2 days after implantation, and then we administered warfarin sodium daily. We examined, both preoperatively and postoperatively, the serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, whole blood hemoglobin, hematocrit, prothrombin time (PT), partial thromboplastin time (PTT) and the plasma hemoglobin. We also recorded the assisted flow rate and the hemodynamic parameters of the animals. After IVAD implantation, the international normalized ratio (INR) was monitored and maintained in the range of 3.5-4.0. Postoperatively, when any device-related problems developed, we euthanized the animals and performed autopsy.
Results
After IVAD implantation, the 5 calves lived for 1, 6, 3, 12 and 21 days, respectively. Three of them were euthanized due to mechanical problems such as electrical shorts, and the other calves died suddenly due to blood leakage at the outflow tract on postoperative day 21 and graft disconnection on postoperative day 3, respectively. Autopsy was performed in all the animals and there was no evidence of thromboembolism or hemorrhage in the kidney, liver or lungs. There was also no evidence of thrombosis on the valve, blood sac or inflow/outflow tract. Hematologic and chemical examinations revealed mild hemolysis in the early postoperative period, which stabilized with minimal hemolysis. There was no organ dysfunction.
Conclusion
Our newly developed Biomedlab® IVAD was feasible for implantation and it functioned well in a calf model. Although there were 3 mechanical problems, we did not find any device-related thrombosis and serious hemolysis. With this encouraging result, it may be possible to perform animal experiments with the final version of IVAD, after correcting the mechanical problems, to evaluate the device's longterm durability and stability.
The incidence of chronic heart failure is increasing due to both aging of the population and the improved medical management, and these factors allow more time for congestive heart failure (CHF) to develop. The therapeutic options are narrowed with the progression of this disease to terminal heart failure and cardiac transplantation then becomes an option, albeit an option of last resort.1)2) Although the recently developed ventricular assist technology serves as a bridge to transplantation, which permits cardiac rehabilitation, improvement of the heart and end-organ function and enhancement of the pre-transplant status, the hematologic effect of ventricular assist systems has not been clearly determined.
In this study, we developed a new Biomedlab® electro-mechanical implantable ventricular assist device (IVAD) that is characterized by pulsatile blood flow assistance, smaller and lighter dimensions than that of its competitors and it is a wearable system (Table 1).3) We performed in vivo experimentation to evaluate the durability and safety of this device as well as its hematologic effects.
The Biomedlab® IVAD is a ventricular assist device that is specifically designed to take over the pumping function of the left ventricle. The Biomedlab® IVAD consists of an implantable blood pump that contains an electric motor, and a blood sac and a driveline (Fig. 1, 2). The driveline connects to a controller and to batteries, which provide power to the pump. The system also includes a display module that displays pump information. The blood pump contains 2 mechanical Medtronic Hall Prosthetic valves (Medtronic Inc. USA) as the inflow and outflow valves. The entire device weighs about 810 grams with dimensions of 97×59 mm. The blood sac is constructed of polyurethane and it collapses for blood pumping when pushed by the motor. For inflow and outflow, 2 polyurethane tubes (3/16 inch) were connected to the implantable pump and the tubes were extended with using a woven Dacron vascular graft (Vascutek Ltd, Renfrewshire, Scotland) for aortic anastomosis.
The animals used in this study were treated in compliance with the "Guidelines for the care and Use of laboratory animals" prepared by the Institute of Laboratory Animal Resources, the National Research Council, and as published by the National Academy Press.
Five calves weighing 60-80 kg were anesthetized (ketamine; 30 mg/kg administered intramuscularly, and isoflurane for maintenance of anesthesia; 2-4% inhalation) and the calves were mechanically ventilated after endotracheal intubation. With monitoring the blood pressure and electrocardiography, a left lateral thoracotomy was done with removal of the 5th rib and another left upper paramedian abdominal incision was made and dissection was performed to the preperitoneal space for creating pockets for implanting the IVAD. After injecting 10,000 units of heparin, the descending thoracic aorta below the level of the azygous vein was clamped with a Satinski-side biting clamp and the prepared Vascutek graft was anastomosed with continuous 4-0 Prolene (Ethicon, Inc. USA) suture for the outflow graft. The pericardium was incised and cradled. After injection of another 14,000 units of heparin, an arterial cannula was inserted into the proximal descending thoracic aorta and a venous cannula was inserted into the right atrium through the atrial appendage. Normothermic cardiopulmonary bypass was instituted and the left ventricle was lifted by hand and the left ventricular apex was widely opened with a scalpel. Four 3-0 Prolene mattress sutures buttressed with felt were sutured at the four corners of the left ventricular (LV) apex and the inflow cuff was sutured with 12 mattress Prolene buttressed with felt. The IVAD was prepared to place in the preperitoneal cavity after filling the blood sac and cannulae with saline. The inflow cannula was prepared to insert from the prepared preperitoneal pocket and diaphragm hole. The inflow cannula tip was inserted into the left ventricule through the inflow cuff with removing the air. The control tube with power cable and vent line was connected to the control device.
We implanted the Biomedlab® IVAD (Version 1.7) into the pre-peritoneal cavity in 5 calves. The inflow cannula was inserted through the left ventricular apex with the user-made inlet tip secured by a plastic cuff sewn onto the epicardial space, and the outflow tract was anastomosed to the descending thoracic aorta using a short segment of dacron graft. The diagram of the blood pump, the inlet and the outlet cannulae are shown in Fig. 1 and 2. The pump flow was monitored using an ultrasonic flow meter.
After implantation of the IVAD, the calves were awaked and their endotracheal tubes were removed at the operating room (Fig. 3). Early after the operation, the IVAD pump flow, the inflow and outflow pressure, the blood pressure, heart rate, pulmonary artery pressure and central venous pressure were continuously monitored. The IVAD flow and arterial pressure were continuously monitored from postoperative day 1. Intravenous heparin was administered continuously for 2 days after implantation, and then warfarin sodium was administered daily to a target range between 3.0 and 4.0. We measured, both preoperatively and postoperatively, the serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, whole blood hemogloin, hematocrit, prothrombin time (PT), partial thromboplastin time (PTT) and plasma hemoglobin. Postoperatively, when we found device-related problems, we then we euthanized the animals and performed autopsy. The removed devices were grossly observed, after gentle washing with saline, for whether there was a manufacture defect or thrombus in the blood contact area. Three points of the luminal side of the sac were evaluated with scanning electron microscopy for intimal proliferation.
The characteristics and outcomes of the five calves used in the experiments are shown in Table 2. After IVAD implantation, the 5 calves lived for 1, 6, 3, 12 and 21 days, respectively. Three of them were euthanized due to mechanical problems such as electrical shorts, and the other 2 calves died suddenly due to blood leakage at the outflow tract on postoperative day 21 and graft disconnection on postoperative day 3, respectively.
Hematologic and chemical studies revealed mild hemolysis in the early postoperative period and this was stabilized with minimizing the hemolysis. However, there was no organ dysfunction seen on the blood chemistry (Table 3, 4).
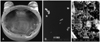
Autopsy was performed in all animals and there was no evidence of thromboembolism or hemorrhage in the kidney, liver or lungs. Post-explant examination of the pump also showed no evidence of thrombosis on the valve, blood sac or inflow/outflow tract (Fig. 3). However, there were small thrombi at the junction of the devices and the prosthetic inflow and outflow valves. On scanning electron microscopic examination, some platelets deposited with fibrin were seen at the inflow side of the blood sac; however, there were significant cellular and fibrin deposits at the outflow area of the blood sac (Fig. 4).
As the number of patients with congestive heart failure increases, the use of left ventricular assist device (LVAD) in these patients is a growing issue worldwide. Many experimental and clinical studies have reported on LVAD implantation in patients with end-stage heart failure and some of these studies are still going on. One of the complications that can occur, although it is not common, with these LVADs is thrombosis within the left ventricle and adjacent to the device's inflow conduit, which may lead to inflow obstruction and multiple thromboembolic events in other organs.4-8) Axial-flow pumps seem to result in new hemodynamic and hematologic characteristics that are not completely understood. The space between the pump inflow conduit and the left ventricular wall is thought to be vulnerable to thrombus formation. In this space, turbulent or stagnant flow may occur, and especially when filling of the left ventricle is impaired, as in patients with right-sided cardiac dysfunction. In addition, because LVAD recipients are often at a high risk for bleeding related to recent surgery or organ dysfunctions, it is difficult to achieve optimal anticoagulation. Since the LVADs are used not only as a bridging therapy to transplantation, but also as a final therapy in recent days,9-13) it seems very important to determine their systemic hematologic effects.
Current knowledge of thrombosis at biomaterial surfaces includes an understanding that protein adsorption, platelet adhesion and fibrin formation are each affected in their own ways by factors such as fluid mechanics and the biomaterial properties.8) The biological series of events that occur with blood contacting biomaterials is well documented and this has led to antiplatelet and anticoagulation therapy, which is now commonly used. Although many advances of anti-coagulation protocols and newer LVADs have been developed, thrombotic complications still occur that can cause patient mortality.
Another major complication related to LVADs is bleeding. 14-16) Bleeding, which occurred in 68% of the LVAD patients in the mechanical circulatory support device (MCSD) database,17) may be due to excess anticoagulation that was originally intended to prevent thrombosis.
In this experiment, we examined the changes of the postoperative hematologic laboratory findings as well as those of the organs and the explanted blood pump to evaluate the effects of thrombosis and hemolysis. There was no evidence of thrombosis or hemolysis in the explanted blood pump and cannulas, as well as in other organs. However, there are several limitations in this experiment. First, we evaluated only five calves, and this is a very small sample size for achieving reliable results. Second, since most of the calves died within a week, the mean values of the laboratory results are also not convincing. However, it may be said that there was no significant hemolytic and thrombotic complications that occurred in the early postoperative periods. Therefore, further experimentation with a longer period and more subjects is necessary to confirm these results before conducting clinical trials. In addition, mechanical problems such as leakage of the devices and connector breakage should be corrected.
We successfully performed a pre-clinical study of a newly developed Biomedlab® IVAD in a calf model. Although there were 3 mechanical problems, no thrombosis or hemolysis occurred in this study. There was only minimal hemolysis in the early postoperative periods and no evidence of thromboembolism in the major organs. However, significant research and development is still necessary to reduce the intimal proliferation at the inner surface of the blood sac. Since this experiment is limited by the small number of animals and their short duration of survival, further animal research with more subjects and a longer study period may be necessary to confirm our results.
Figures and Tables
Fig. 4
Gross and microscopic findings of removed blood sac. Removed blood sac for the gross findings (A) and scanning electron microscopic findings at the inflow (B) and outflow (C) tracks from calf #4 (POD 13). POD: postoperative day.
References
1. Lee S, Park YH, Lim SH, Kwak YT, Kim H, Chang BC. Successful mechanical circulatory support as a bridge to transplantation. Asian Cardiovasc Thorac Ann. 2007. 15:243–245.
2. Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995. 222:327–338.
3. Cho HS, Kim WG, Lee WY, et al. Development and evaluation of a novel electro-mechanical implantable ventricular assist system. J Biomed Eng Res. 2001. 22:349–358.
4. Meuris B, Arnout J, Vlasselaers D, Schetz M, Meyns B. Long-term management of an implantable left ventricular assist device using low molecular weight heparin and antiplatelet therapy: a possible alternative to oral anticoagulants. Artif Organs. 2007. 31:402–405.
5. Hetzer R, Weng Y, Potapov EV, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004. 25:964–970.
6. Delgado R 3rd, Frazier OH, Myers TJ, et al. Direct thrombolytic therapy for intraventricular thrombosis inpatients with the Jarvik 2000 left ventricular assist device. J Heart Lung Transplant. 2005. 24:231–233.
7. Rothenburger M, Wilhelm MJ, Hammel D, et al. Treatment of thrombus formation associated with the MicroMed DeBakey VAD using recombinant tissue plasminogen activator. Circulation. 2002. 106:Suppl. I189–I192.
8. Yamanaka H, Rosenberg G, Weiss WJ, Snyder AJ, Zapanta CM, Siedleki CA. Short-term in vivo studies of surface thrombosis in a left ventricular assist system. ASAIO J. 2006. 52:257–265.
9. Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002. 21:516–521.
10. Frazier OH, Myers TJ, Westaby S, Gregoric ID. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003. 237:631–636.
11. Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999. 68:734–741.
12. Roman J, Jeevanadam V. Destination therapy with ventricular assist device. Cardiology. 2004. 101:104–110.
13. Boehmer JP. Device therapy for heart failure. Am J Cardiol. 2003. 91:53D–59D.
14. Hunt SA, Frazier OH. Mechanical circulatory support and cardiac transplantation. Circulation. 1998. 97:2079–2090.
15. McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical experience with 111 Thoratec ventricular assist devices. Ann Thorac Surg. 1999. 67:1233–1239.
16. Minami K, El-Banayosy A, Sezai A, et al. Morbidity and outcome after mechanical ventricular support using Thoratec, Novacor, and HeartMate for bridging to heart transplantation. Artif Organs. 2000. 24:421–426.
17. Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the international society for heart and lung transplantation: second annual report, 2004. J Heart Lung Transplant. 2004. 23:1027–1034.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download