Abstract
Background
The aim of the present study was to prepare hydroxyapatite (HA) and then characterize its effect on bone integration in a rabbit tibial defect model. The bone formation with different designs of HA was compared and the bony integration of several graft materials was investigated qualitatively by radiologic and histologic study.
Methods
Ten rabbits were included in this study; two holes were drilled bilaterally across the near cortex and the four holes in each rabbit were divided into four treatment groups (HAP, hydroxyapatite powder; HAC, hydroxyapatite cylinder; HA/TCP, hydroxyapatite/tri-calcium phosphate cylinder, and titanium cylinder). The volume of bone ingrowth and the change of bone mineral density were statistically calculated by computed tomography five times for each treatment group at 0, 2, 4, 6, and 8 weeks after grafting. Histologic analysis was performed at 8 weeks after grafting.
Results
The HAP group showed the most pronounced effect on the bone ingrowth surface area, which seen at 4, 6, and 8 weeks after graft (p < 0.05). On comparing the change of bone mineral density the bone ingrowth surface area among the 4 groups, there were no statistically significant differences among the groups found for any period (p > 0.05). On histological examination, the HAP group revealed well-recovered cortical bone, but the bone was irregularly thickened and haphazardly admixed with powder. The HAC group showed similar histological features to those of the HA/TCP group; the cortical surface of the newly developed bone was smooth and the bone matrix on the surface of the cylinder was regularly arranged.
Conclusions
We concluded that both the hydroxyapatite powder and cylinder models investigated in our study may be suitable as a bone substitute in the rabbit tibial defect model, but their characteristic properties are quite different. In contrast to hydroxyapatite powder, which showed better results for the bone ingrowth surface, the hydroxyapatite cylinder showed better results for the sustained morphology.
Extensive bone injuries that require grafting typically involve one of two kinds of bone material: autograft or allograft.
Large osseous defects pose a significant treatment challenge to orthopaedic surgeons, and nonunion is also common. Although the gold standard for treating large osseous defects is autologous bone grafting, this procedure is constrained by the inability to obtain large structural grafts.
Nevertheless, an autologous cortico-cancellous bone graft is the most commonly used type of graft for various orthopedic conditions. However, this type of graft is limited in quantity and it may not be adequate for filling large defect. Harvesting an autologous graft has its own limitations. It requires another operation with additional loss of blood and a prolonged anesthetic time. It may also lead to donor site complications like neurovascular injury, infection, hematoma formation and chronic pain.1,2)
To overcome these limitations, synthetic grafts are preferred in place of autologous grafts. Various studies have proved the efficacy of hydroxyapatite as a substitute for an autologous graft.1-13) Hydroxyapatite appears to be the most appropriate when a bone substitute with some mechanical strength is needed, as hydroxyapatite is composed of the natural minerals found in human bone and it approximates the natural structure of cancellous bone.14) Hydroxyapatite is a biocompatible material with osteoconductive properties. It is available in various forms and the bone formation and graft incorporation varies according to the type. Unfortunately, bone-graft substitutes consisting solely of particles are mechanically weak and the particles may migrate from the graft site before ingrowth of new bone tissue secures them in place.
The aim of the present study was to prepare hydroxyapatite and characterize its effect on bone integration in a tibial defect rabbit model. We investigated the bone formation with different designs of hydroxyapatite, and the bony integration of several graft materials was qualitatively investigated by radiologic and histologic studies.
Ten New Zealand white rabbits that were approximately 15 weeks old at the beginning of the experiment and that weighed 2 kg to 2.5 kg were included in the study. The protocol for the animal experiment was approved by the Institutional Animal Care and Use Committee of Dong-A University. The rabbits were handled according to the guidelines established for animal care at the center. Each rabbit had free access to both sterile water and standard rodent soft chow ad libitum.
In this study, two designs of hydroxyapatite were investigated: hydroxyapatite powder (HAP) and a hydroxyapatite cylinder (HAC). The HAP was fabricated by calcining hydroxyapatite for 1 hour at 900℃, with a resulting particle size between 500 µm to 700 µm. The HAC was produced with these powders by slip casting in a mold that had longitudinally oriented porosity (3 mm diameter × 8 mm length). In addition, a hydroxyapatite-tri-calcium phosphate (HA-TCP) mixture cylinder (3 mm diameter × 8 mm length) and a titanium cylinder (3 mm diameter × 7 mm length) were prepared at a similar size (Fig. 1). The hydroxyapatite composite cylinders were prepared with compositions of 20% TCP. The obtained shaped samples were treated for 1 hour at 1,250℃ and then they were sterilized at 120℃ for 20 minutes before implantation.
The surgical procedures were done after intramuscular administration of ketamine (100 mg/kg). Prior to surgery, the skin was shaved and next cleaned with a mixture of iodine and 70% ethanol. The upper 1/3 of the tibia was exposed by making a 2 cm midline incision through the skin, fascia and periosteum. Two holes, each 3.5 mm in diameter, were drilled bilaterally across the near cortex and the four holes in each rabbit were divided into four treatment groups. The skin was sutured layer-by-layer after the HAP, HAC, HA/TCP cylinders and titanium cylinder were pressed into their respective holes (Fig. 2). The animals were given 0.05 mg/kg buprenorphine subcutaneously every 12 hours for the first 48 hours after the operation. Within 2 - 3 days, the animals resumed normal ambulation and did not show signs of pain or distress. Radiologic analysis was performed every two weeks and all the animals were sacrificed eight weeks after implantation for histologic analysis.
We recorded the changes of the bone ingrowth surface area around the graft material, as assessed by tomogram resolution (FLEX™ for platform X-O™, GMI, Northridge, CA, USA) (Fig. 3). This may indicate the affinity of the bone ingrowth for the surface of the graft material. We measured all the surface areas as the number of voxel (volumetric pixel) faces at the interface in 3 D multiplied by the voxel dimension squared. The volume was normalized and the surface area density was recorded in units of mm3. The bone mineral density of the graft material was also reported in the same manner. We normalized the volume and we recorded the bone mineral density in units of mg/ml. The volume of the bone ingrowth and the bone mineral density were calculated five times for each treatment group at 0, 2, 4, 6, and 8 weeks after grafting.
Histologic analysis was performed at 8 weeks after grafting. The retrieved specimens of the rabbit tibia were stripped of soft tissues, fixed in 4% buffered for undecalcified bone processing. The samples were then dehydrated in graded series of alcohol solution until the absolute was reached. Finally, they were embedded in polymethylmethacrylate.
The blocks were sectioned along a plane parallel to the major axis of the cylinder using a Micro-grinding machine (MG4000, EXAKT Apparatebau GmbH, Norderstedt, Germany). A series of 50-µm-thick sections were obtained to allow comparison between the areas under investigation. The sections were stained with Hematoxylin-Eosin. Routine histology and histomorphometric analyses were performed using transmission light microscopy (Axioskop Carl Zeiss GmbH, Jena, Germany) and image-analysis software (KS 300; Kontron Electronic GmbH, Munchen, Germany).
The volume of bone ingrowth and the bone mineral density at 0, 2, 4, 6, and 8 weeks after grafting were expressed as means ± standard deviations. Group comparisons were made using analysis of variance with the Bonferroni correction for multiple comparisons. In all cases, a p-value of less than 0.05 was deemed statistically significant.
All rabbits were kept in individual cages throughout the experimental periods. No histopathologic features of graft-versus-host disease or immune rejection were observed in any of the treatment group.
The HAP group showed the most pronounced effect on the bone ingrowth surface area, which seen at 4, 6, and 8 weeks after graft (p < 0.05). The earliest difference was seen at 4 weeks after grafting when the HAP group had an average volume of 118.97 ± 7.25 mm3, whereas the HAC group (86.52 ± 4.92 mm3), HA/TCP group (78.12 ± 4.91 mm3), and titanium group (73.07 ± 6.76 mm3) were relatively poorly healed, as measured by the volume of the bone ingrowth surface area (p < 0.05) (Fig. 4). Although there was a slightly higher trend for an increased bone ingrowth surface area in the HAC group as compared to that in the HA/TCP and titanium groups at the 4th and 6th weeks after grafting, there were no statistically significant differences found for any period between the three groups (Fig. 4). Regarding the changes of bone mineral density, there were no statistically significant differences found for any period between the four groups (Fig. 5).
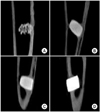
On histologic examination, the HAP group 8 weeks after grafting demonstrated well recovered cortical bone, but the bone was irregularly thickened and haphazardly admixed with powder. The arrangement of the bone matrix was slightly disrupted and the cortical surface of the bone was coarse. The HAC group showed similar histological features as those of the HA/TCP group: the hole was entirely blocked with the cylinder and cortical bone ingrowth tightly surrounded the cylinder. The cortical surface of the newly developed was smooth and the new bone matrix around the cylinder was regularly arranged. Although the titanium group showed similar histological features of bone ingrowth surrounding the cylinder as the HAC and HA/TCP groups, in the titanium group, the cortical layer above the cylinder did not recover as well (Fig. 6) and this was also shown on computed tomography (Fig. 7).
Hydroxyapatite is a mineral that is a naturally occurring form of calcium apatite with the formula Ca5(PO4)3(OH), but this is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two molecules. Seventy percent of bone is made up of the inorganic mineral hydroxyapatite. Carbonated calcium-deficient hydroxyapatite is the primary mineral that dental enamel and dentin are made of. Hydroxyapatite crystals are also found in the small calcifications within the pineal gland and other structures; this is known as corpora arenacea or "brain sand."6) Hydroxyapatite can be found in the teeth and bones within the human body. Thus, it is commonly used as a filler to replace amputated bone or as a coating to promote bone ingrowth into prosthetic implants. Although many other phases exist with a similar or even identical chemical makeup, the body responds much differently to them. Coral skeletons can be transformed into hydroxyapatite under high temperatures; their porous structure allows relatively rapid ingrowth at the expense of the initial mechanical strength. The high temperature also burns away any organic molecules such as proteins, which prevents graft-versus-host disease and rejection. Many modern implants, e.g., hip replacements and dental implants, are coated with hydroxyapatite. It has been suggested that this may promote osseointegration, and there is strong evidence to support this idea.6)
There have been some reports of successful integration of hydroxyapatite in dental surgery,15-18) and less frequently, of the use of hydroxyapatite in orthopedics.8) Radiographic and biomechanical assessments of hydroxyapatite in a canine metaphyseal defect model were performed by Sartoris et al.19) who claimed that this bone substitute was even better than autogenous bone grafts for the radiographic and mechanical parameters. In another study of hydroxyapatite in canine metaphyseal defects, Holmes et al.17) reported that at 12 months, two-thirds of the hydroxyapatite surface was covered with appositional bone. Martin et al.20) reported rapid bony integration of hydroxyapatite in a canine cancellous bone defect, but they found the material less suitable for cortical defects due to its low mechanical strength. Bucholz et al.4) performed a prospective randomized comparison of hydroxyapatite and autogenous bone grafts in 40 tibial plateau fractures. Since there were no differences in the outcome between the two groups, the authors suggested that this porous hydroxyapatite is as effective as an autograft in these specific circumstances. Ripamonti21) found new bone formation after heterotopic implantation of hydroxyapatite in adult primates and they concluded that this bone substitute represents a biological alternative to autogenous bone grafts for the controlled initiation of bone formation in humans.
In our study, hydroxyapatite allograft integration occurred after an initial immunologic response with the formation of new bone inside the allograft, as well as with direct bone bridging. A different type of bone remodeling took place in the empty cavities, where the initial blood clot was reorganized by mesenchymal cells, followed by ingrowth of fibroblasts and osteoblasts, with spontaneous osteoneogenesis from the edges to the center of the cavities. The latter finding contradicts the results published by Katthagen;22) they found that 6 mm drill holes in the rabbit femoral condyle remained empty with no regeneration of bone for months. However, in another study, even 10 mm circular metaphyseal defects in dogs were reported to be filled with spontaneously regenerated bone within 3 weeks.23) In the present study, no histopathologic features of graft-versus-host disease or immune rejection were observed in any of the groups. The results suggested that accelerated bone healing, as determined by CT measurement of bone ingrowth and the histological pathology, were obtained in the hydroxyapatite and hydroxyapatite composite groups.
Our study supports the use of hydroxyapatite as an alternative to autologous cancellous bone graft. It avoids the need for harvesting bone and it does not cause any allergic or inflammatory reactions. CT analysis showed excellent bone integration after 8 weeks in all the treatment groups, except the titanium cylinder group, which demonstrated a less well-recovered cortical layer above the cylinder. Although hydroxyapatite powder showed the most pronounced effect on the bone ingrowth surface area, the hydroxyapatite cylinder showed a better result on the histologic analysis. The application of the latter is simple and hydroxyapatite cylinders or blocks can easily be modeled to the required size and to many kinds of orthopedic situations.
The recent developments of CT systems allow the use of this technology in vivo;24,25) hence, it could be used noninvasively in follow-up studies. This will increase the quality of the data because interventions can be assessed on an animal-specific basis. Each animal acts as its own control, allowing the examination of individual response characteristics, and thereby eliminating some of the problems of cross-sectional studies. The possibility of monitoring the scaffold morphology and bone ingrowth into these scaffolds has been recently demonstrated. Because of their longitudinal design, these studies required a lesser number of animals than would have been needed in a cross-sectional study. Nevertheless, the full potential of assessing scaffold-bone interaction using in vivo microCT has yet to be realized. Direct microCT-based image analysis allows the accurate quantification of scaffold porosity, surface area and 3 D measures such as pore size, pore distribution and strut thickness; furthermore, it allows for a precise measurement of bone growth into the scaffold and onto its surface. The aim of this paper is to present quantitative analysis of bone ingrowth volume and bone mineral density on a microCT analysis. It was not focused on imaging and the qualitative aspects of bone research, and the study did not address the assessment of graft architecture and how it interacts with bone tissue. Further qualitative studies using microCT to assess the graft architecture and its interaction with bone tissue are needed.
We concluded that both the hydroxyapatite powder and cylinder model investigated in our study may be suitable as a bone substitute in the tibial defect rabbit model, yet their characteristic properties are is quite difffferent. In contrast to hydroxyapatite powder, which showed better results on the bone ingrowth surface, the hydroxyapatite cylinder showed better results for the sustained morphology.
Hydroxyapatite powder may be used as a simple filler in sites that do not have stress concentrated on them. A hydroxyapatite cylinder may be used as substitution for cortical bone, but the strength and stiffness of the grafted repair should be assessed.
Further studies should be performed to compare this version of hydroxyapatite to other bone substitutes, and possibly in combination with other osteogenic proteins or growth factors.
Figures and Tables
Fig. 1
(A) Photographs of the hydroxyapatite powder and (B) the types of graft substituted cylinders (1. hydroxyapatite cylinder [HA], 2. hydroxyapatite/tri-calcium phosphate cylinder [HA/TCP], 3. titanium cylinder).

Fig. 2
The diagram of 4 holes (divided into four treatment groups). HAP: Hydroxyapatite powder, HAC: Hydroxyapatite cylinder, HA/TCP: Hydroxyapatite/tri-calcium phosphate cylinder, Titanium: Titanium cylinder.
Fig. 4
Comparison of the bone ingrowth surface area in each group over an 8 weeks period. Significant differences were found at 4, 6, and 8 weeks after grafting between the HAP group and the other groups (p < 0.05). HAC: Hydroxyapatite cylinder, HAP: Hydroxyapatite powder, HA/TCP: Hydroxyapatite/tri-calcium phosphate cylinder, Titanium: Titanium cylinder.

Fig. 5
Comparison of the change of bone mineral density among the 4 groups over an 8 weeks period. There were no statistically significant differences found at any period between the four groups (p > 0.05). HAC: Hydroxyapatite cylinder, HAP: Hydroxyapatite powder, HA/TCP: Hydroxyapatite/tri-calcium phosphate cylinder, Titanium: Titanium cylinder.

Fig. 6
Comparison of the histological findings of the hematoxylin and eosin-stained sections among the 4 groups (A: hydroxyapatite powder [HAP], B: hydroxyapatite cylinder [HAC], C: hydroxyapatite/tri-calcium phosphate cylinder [HA/TCP], D: titanium cylinder) 8 weeks after graft. The cortical bone is irregularly thickened and haphazardly admixed with HAP (boxed area), in contrast to that of the HAC and HA/TCP groups. *The titanium group demonstrated a less well-recovered cortical layer above the cylinder.

Fig. 7
Comparison of the CT findings among the 4 groups (A: hydroxyapatite powder, B: hydroxyapatite cylinder, C: hydroxyapatite/tri-calcium phosphate cylinder, D: titanium cylinder) 8 weeks after grafting. In contrast to the other groups, the titanium group (D) demonstrated a less well recovered cortical layer above the cylinder.
References
1. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996. (329):300–309.

2. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res. 1997. (339):76–81.

3. Agrillo U, Mastronardi L, Puzzilli F. Anterior cervical fusion with carbon fiber cage containing coralline hydroxyapatite: preliminary observations in 45 consecutive cases of soft-disc herniation. J Neurosurg. 2002. 96:3 Suppl. 273–276.

4. Bucholz RW, Carlton A, Holmes R. Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Clin Orthop Relat Res. 1989. (240):53–62.

5. Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine (Phila Pa 1976). 2000. 25(5):563–569.
6. Holmes R, Mooney V, Bucholz R, Tencer A. A coralline hydroxyapatite bone graft substitute: preliminary report. Clin Orthop Relat Res. 1984. (188):252–262.
7. Keating JF, Hajducka CL, Harper J. Minimal internal fixation and calcium-phosphate cement in the treatment of fractures of the tibial plateau: a pilot study. J Bone Joint Surg Br. 2003. 85(1):68–73.
8. Sartoris DJ, Gershuni DH, Akeson WH, Holmes RE, Resnick D. Coralline hydroxyapatite bone graft substitutes: preliminary report of radiographic evaluation. Radiology. 1986. 159(1):133–137.

9. Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement: a prospective, randomised study using Norian SRS. J Bone Joint Surg Br. 2000. 82(6):856–863.
10. Thalgott JS, Fritts K, Giuffre JM, Timlin M. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine (Phila Pa 1976). 1999. 24(13):1295–1299.

11. Uchida A, Nade SM, McCartney ER, Ching W. The use of ceramics for bone replacement: a comparative study of three different porous ceramics. J Bone Joint Surg Br. 1984. 66(2):269–275.

12. Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990. 72(2):298–302.

13. Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours: clinical results. J Bone Joint Surg Br. 2000. 82(8):1117–1120.
15. Boyne PJ. Implant dentistry forefront '85: design and methods. J Oral Implantol. 1986. 12(3):333–337.
16. Finn RA, Bell WH, Brammer JA. Interpositional "grafting" with autogenous bone and coralline hydroxyapatite. J Maxillofac Surg. 1980. 8(3):217–227.

17. Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects: a histometric study. J Bone Joint Surg Am. 1986. 68(6):904–911.

18. Piecuch JF, Topazian RG, Skoly S, Wolfe S. Experimental ridge augmentation with porous hydroxyapatite implants. J Dent Res. 1983. 62(2):148–154.

19. Sartoris DJ, Holmes RE, Tencer AF, Mooney V, Resnick D. Coralline hydroxyapatite bone graft substitutes in a canine metaphyseal defect model: radiographic-biomechanical correlation. Skeletal Radiol. 1986. 15(8):635–641.

20. Martin RB, Chapman MW, Holmes RE, et al. Effects of bone ingrowth on the strength and non-invasive assessment of a coralline hydroxyapatite material. Biomaterials. 1989. 10(7):481–488.

21. Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 1991. 73(5):692–703.

22. Katthagen BD. Bone regeneration with bone substitutes: an animal study. 1986. Berlin: Springer-Verlag;47–50.
23. Schweiberer L. Experimental studies on bone transplantation with unchanged and denaturated bone substance: a contribution on causal osteogenesis. Hefte Unfallheilkd. 1970. 103(1):1–70.
24. David V, Laroche N, Boudignon B, et al. Noninvasive in vivo monitoring of bone architecture alterations in hindlimb-unloaded female rats using novel three-dimensional microcomputed tomography. J Bone Miner Res. 2003. 18(9):1622–1631.

25. Waarsing JH, Day JS, van der, et al. Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone. 2004. 34(1):163–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download