Abstract
Purpose
This study assessed the incidence and cancer rate of probably benign lesions detected on bilateral whole-breast screening ultrasound (US), which corresponded to US Breast Imaging Reporting and Data System (BI-RADS) category 3, and evaluated the proper management of those lesions.
Methods
This study was approved by the Institutional Review Board in our institution, which waived informed patient consent. We retrospectively reviewed US images of 1,666 patients who underwent bilateral whole-breast screening US as a supplemental screening test to negative screening mammography or screening US only. The incidence, clinical course, and cancer rate of screening US-detected probably benign lesions corresponding to US BI-RADS category 3 were investigated, and the size and multiplicity of screening US-detected category 3 lesions were evaluated.
Results
Probably benign lesions corresponding to US BI-RADS category 3 were detected in 689 of 1,666 patients (41.4%) who underwent screening US. Among them, 653 had follow-up US images for at least 24 months, and among these 653, 190 (29.1%) had multiple bilateral category 3 lesions. Moreover, 539 of 1,666 patients (32.4%) had lesions ≤1 cm in size and 114 of 1,666 (6.8%) had lesions >1 cm (median, 0.82 cm; range, 0.3–4.2 cm). Four of the 653 patients (0.6%) showed suspicious interval changes and were categorized into BI-RADS category 4. Biopsy analysis confirmed only one lesion as invasive ductal carcinoma at the 6-month follow-up; another lesion was an intraductal papilloma and the remaining two were fibroadenomas. Overall cancer rate of the screening US-detected BI-RADS category 3 lesions was 0.2%.
The importance of breast cancer screening is undeniable. Early detection of cancer significantly reduces mortality and often enables breast-conserving surgery [1]. Although mammography is the gold standard for breast cancer screening in the general population, dense breast composition may make breast cancer more difficult to detect. In addition to mammography, ultrasound (US) has been widely used in the screening of breast cancer because of the ability of breast US to depict an occult malignancy in women with negative mammography results [2345]. Many studies have shown that US increases the sensitivity of screening if used as an adjunct to mammography in women having dense breasts [45678910111213]. The addition of whole-breast US to screening mammography in women with an elevated risk of breast cancer increases cancer detection by 4.2 cancers per 1,000 women [12]. Moreover, whole-breast screening US helps diagnose more women with nonpalpable invasive cancers, especially when no other cancers are detected in them by using other screening modalities [6]. The breasts of Asian women are relatively smaller and denser than the fatty, larger breasts of Western women [141516], and screening US has become the preferred routine screening tool for patients with dense breasts.
One of the biggest issues is the unacceptably high frequency of category 3 lesions, according to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS), detected on US in asymptomatic patients [17]. Previous studies have shown that the incidence of probably benign lesions in bilateral whole-breast screening US can be as high as 36.9% and 33.6% in the general population [418]. We have been recommending short-term US follow-up for all patients with these benign-looking lesions, but it greatly increases treatment cost as well as the false-positive rates.
Despite the increased number of whole-breast screening US tests and screening US-detected category 3 lesions, a few studies have evaluated the outcome and risk of category 3 lesions detected on screening US [192021]. One recent paper based on the American College of Radiology Imaging Network 6666 trial results documented the outcome of screening US-detected category 3 lesions, but the study was performed in women with increased breast cancer risk and dense breasts [19]. Therefore, we considered investigating the outcome of the BI-RADS category 3 lesions detected on screening US with long-term follow-up and drafting management guidelines for these lesions.
The purpose of this study was to assess the frequency and cancer rate of probably benign lesions detected on whole-breast screening US, which corresponded to US BI-RADS category 3 lesions, and to evaluate the proper management of such lesions.
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB number: SMC 2012-02-019), which waived informed patient consent.
We retrospectively reviewed the US images of 1,666 consecutive patients who underwent bilateral whole-breast screening US as a supplemental screening to a negative screening mammography (BI-RADS category 1 or 2) (n=1,621), or who underwent whole-breast screening US only (n=45) at the health promotion center of our hospital. Among the 1,621 patients who had additional whole-breast screening US with negative screening mammography results, 1,258 (77.6%) had a dense breast composition (ACR BI-RADS grade 3, 4) and 363 (22.4%) had a fatty composition (ACR BI-RADS grade 1, 2). The age of the patients ranged from 30 to 76 years (median age, 49.5 years). Among the 45 patients who had undergone whole-breast screening US only, 27 (60.0%) were aged ≤40 years and 18 (40.0%) were over 40 years of age but did not want to undergo mammography for fear of pain or a recent negative mammography result obtained at another facility.
The women enrolled in this study were healthy and asymptomatic subjects with an average risk of breast cancer and mainly dense breasts. Only 10 of the 1,666 patients (0.6%) had a personal history of previous breast cancer, and only 18 (1.1%) had a family history of breast cancer.
All patients underwent bilateral whole-breast screening US performed by one of the five board-certified breast-dedicated radiologists. These radiologists were aware of the results of the screening mammography and had reviewed the mammograms before performing the breast US. We used the HDI 5000 US scanner (Advanced Technology Laboratories, Bothell, USA) or the LOGIQ 700 US scanner (GE Medical Systems, Milwaukee, USA) equipped with a 12- to 5-MHz linear-array transducer.
Two board-certified breast radiologists (with 5 years and 12 years of experience in breast US) who did not perform the whole-breast screening US retrospectively reviewed the 1,666 US images. In cases with positive US findings, the lesions were analyzed according to the BI-RADS lexicon and categorized according to the BI-RADS final assessment category after reaching consensus. At the time of the review, the readers were blinded to the mammographic findings, which were categorized as BI-RADS 1 or 2, or the result of prior screening US examinations. The US criteria used to define BI-RADS category 3 lesions were a solid mass with circumscribed margins, oval shape and horizontal orientation, most likely a fibroadenoma, nonpalpable complicated cysts, and clustered microcysts. According to the management guidelines of the ACR, these lesions were to be followed up for 6, 12, and 24 months.
We analyzed the incidence of BI-RADS category 3 lesions detected on whole-breast screening US and evaluated the clinical course of these lesions as well as the cancer rate by analyzing the follow-up clinical and US data of patients with such lesions. We also evaluated the size (≤1 cm vs. >1 cm) and multiplicity (multiple bilateral lesions or not) of the screening US-detected BI-RADS category 3 lesions. Multiple bilateral lesions were defined as lesions with the same US findings in both breasts and multiple lesions in at least one breast.
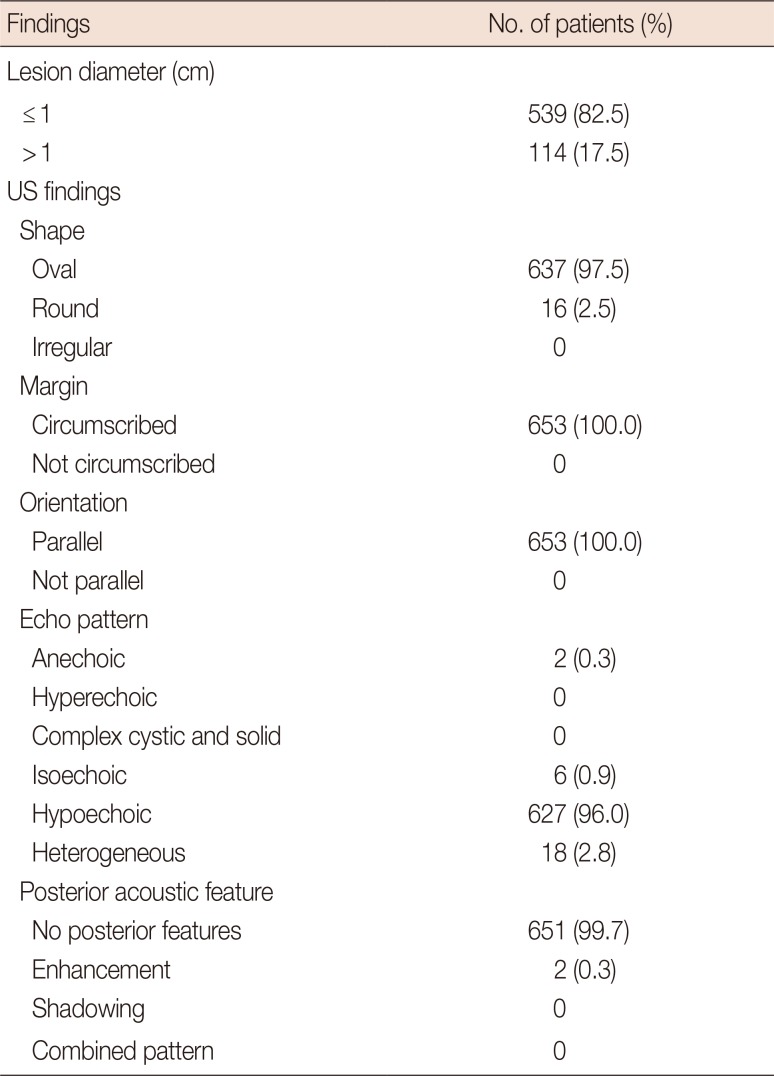
Among the 1,666 patients who underwent bilateral whole-breast screening US, 689 women (41.4%) had category 3 lesions (Figure 1). Follow-up US images for more than 24 months were present in 653 of 689 patients (94.8%). We excluded 36 patients who had either no follow-up US data or a follow-up period <24 months. Finally, we analyzed the clinical courses and cancer rate of the remaining 653 patients. One hundred and ninety patients (29.1%) had multiple bilateral category 3 lesions, and we evaluated the representative lesion in each of those patients. The lesions were ≤1 cm in size in 539 of 1,666 patients (32.4%) and were >1 cm in 114 of 1,666 (6.8%) (median, 0.82 cm; range, 0.3–4.2 cm). Among the 653 patients, 10 had lesions that were complicated cysts and 643 had lesions that were solid masses. The US findings of 653 category 3 lesions are shown in Table 1.
After the 24-month follow-up, lesions in 592 patients (90.7%) showed no interval change or decrease in size, and had converted to BI-RADS category 2 lesions (Figure 2). Lesions in 57 patients (8.7%) showed a subtle change in size, shape, or echogenicity, but no significant interval change defined as a <20.0% interval growth rate; moreover, their US category remained unchanged as category 3 after 24 months (mean follow-up period, 54.6±15.7 months). In total, 31 lesions were biopsied after initial screening US or during follow-up for several reasons, including a request from the physician (n=20) or the patient (n=7) and suspicious interval change (n=4). Four women (0.6%) with US-detected category 3 lesions showed suspicious interval change of morphology or significant increase in the size of the lesions. They had converted to BI-RADS category 4 lesions during the follow-up period. Two of these lesions had morphological changes, one lesion had increased in size, and one lesion had morphological changes and increase in size. Only one lesion was confirmed as an invasive ductal carcinoma at core needle biopsy after a 6-month follow-up US (Figure 3). This patient underwent partial mastectomy, and pathological analysis of the surgical specimen revealed a 1.2 cm sized invasive ductal carcinoma without lymph node metastasis (T1N0M0). The overall cancer rate of patients who had US-detected BI-RADS category 3 lesions was 0.2% (1/653).
The other three lesions, which had converted to category 4 lesions, were confirmed as intraductal papilloma (n=1) and fibroadenomas (n=2) (Figure 4) after US-guided core needle biopsy. Of the two patients with fibroadenomas, one underwent excisional biopsy and the other underwent a follow-up US. The patient with intraductal papilloma underwent a mass excision. The pathological results after surgical excisions were identical to those of the results of core needle biopsies.
Bilateral whole-breast screening US has several drawbacks including the long time to perform bilateral whole-breast US [12], higher expense of US examination than mammography [22], and higher false-positive rates [23]. Moreover, the positive predictive value of biopsy analysis of lesions identified on screening US is 8.9%, whereas it is 22.6% for mammography [12]. The high frequency of BI-RADS category 3 lesions is another problem, which will increase the number of breast US examinations, time for examinations, and costs. The reported rate of BI-RADS category 3 lesions on bilateral whole-breast screening US ranges from 20.2% to 33.6% [181924]. The detection rate of BI-RADS category 3 lesions in our study was 41.4%.
In our screening population, additional cancers were found in 10 of 1,666 patients (0.6%) who underwent bilateral whole-breast screening US. The follow-up results of the screening US-detected BI-RADS category 3 lesions revealed a cancer rate of 0.2% (one of 653 patients), and that patient had a T1N0M0 stage cancer. Barr et al. [19] reported that the overall cancer rate for BI-RADS category 3 lesions was 0.9% (six of 636 lesions) in women with increased breast cancer risk, and Moon et al. [24] reported that the cancer rate of BI-RADS category 3 lesions was 0.2% in their study that included both screening and diagnostic breast US. The results of our study showed much lower cancer rates for BI-RADS category 3 lesions, because we mainly screened women with an average risk of breast cancer. These data indicate that the short-term follow-up US for screening US-detected BI-RADS category 3 lesions did not help detect more cancers. BI-RADS category 3 final assessment of breast US was introduced for the lesions detected on diagnostic evaluations, and not for the lesions detected by bilateral whole-breast screening US. As the present results indicate, it is difficult to expect that the lesions detected on screening US would show the same outcomes as diagnostic US-detected category 3 lesions, which have palpable abnormality, bloody nipple discharge, or mammographic abnormality [2526].
The cancer rate of screening US-detected category 3 lesions in our study (0.2%) is lower than that of Kim et al. [18] who reported a cancer rate of up to 0.8%. However, the latter rate could have possibly reflected the higher percentage of high-risk patients in that study. In their study, 22.1% of the subjects were high-risk women, whereas in our study, the percentage was only 1.7%. They also included 20.0% women who had a personal history of breast cancer; this percentage was only 0.6% in our study. Considering the overall cancer rate of coexisting category 3 lesions in patients with known breast cancer was 11.4% [27], the malignancy potential of category 3 lesions detected on screening of normal healthy women and screening after breast cancer surgery might be different, especially if the percentage of women who have undergone breast-conserving surgery is higher.
Our study has some limitations. First, it was a retrospective analysis of previously acquired US images, and there is a possibility that the actual assessment of the lesions may have differed. Some existing radiologic reports did not follow the BI-RADS reporting system, and they categorized all small-sized solid lesions or multiple, bilateral probably benign nodules as category 2 lesions. Therefore, we had to review all of the images retrospectively and had to reassess the lesions with homogeneous and strict criteria. To overcome interobserver variation, two experienced breast radiologists analyzed the images and reached consensus. Second, this was a cross-sectional analysis of the US findings of whole-breast screening US conducted at one time; some of our patients had undergone previous breast screening US. This could decrease the cancer rate of category 3 lesions. However, the overall cancer rate of screening US-detected lesions was 0.6%, and the incidence of cancer in our study was not lower than that of other previous studies [234682829]. Third, the patients enrolled in our study had not undergone US examination according to the same schedule. Breast US had been conducted three or more times in some patients, while being done only two times in other patients at the end of 24 months or after 24 months. Finally, we tried to identify any specific characteristics such as size, multiplicity, bilaterality, or specific US features that could decrease or increase the risk of malignancy of screening US-detected probably benign lesions. However, that was impossible because only one patient with a category 3 lesion developed cancer during the follow-up period; moreover, the lesion had no specific US feature. A suspicious interval change on the follow-up US examination was the most important finding.
In conclusion, our study has shown that the incidence of lesions corresponding to BI-RADS category 3 of diagnostic breast US was very high on bilateral whole-breast screening US, but the cancer rate was very low. We suggest that in an average-risk population, routine screening US is preferable over short-term follow-up for screening US-detected BI-RADS category 3 lesions.
References
1. Kopans DB. The 2009 U.S. Preventive Services Task Force guidelines ignore important scientific evidence and should be revised or withdrawn. Radiology. 2010; 256:15–20. PMID: 20574081.

2. Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dünser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999; 173:921–927. PMID: 10511149.

3. Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound: a retrospective review. Cancer. 1995; 76:626–630. PMID: 8625156.

4. Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001; 221:641–649. PMID: 11719658.

5. Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US-diagnostic yield and tumor characteristics. Radiology. 1998; 207:191–199. PMID: 9530316.

6. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002; 225:165–175. PMID: 12355001.

7. Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology. 2002; 224:569–576. PMID: 12147858.

8. Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003; 181:177–182. PMID: 12818853.

9. Leconte I, Feger C, Galant C, Berlière M, Berg BV, D'Hoore W, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003; 180:1675–1679. PMID: 12760942.
10. Benson SR, Blue J, Judd K, Harman JE. Ultrasound is now better than mammography for the detection of invasive breast cancer. Am J Surg. 2004; 188:381–385. PMID: 15474430.

11. Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004; 233:830–849. PMID: 15486214.

12. Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008; 299:2151–2163. PMID: 18477782.

13. Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010; 20:734–742. PMID: 19727744.

14. Maskarinec G, Nagata C, Shimizu H, Kashiki Y. Comparison of mammographic densities and their determinants in women from Japan and Hawaii. Int J Cancer. 2002; 102:29–33. PMID: 12353230.

15. Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2005; 14:1986–1990. PMID: 16103449.

16. Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat. 2007; 104:47–56. PMID: 17009106.

17. Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. ACR BI-RADS ultrasound. In : D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology;2013.
18. Kim SJ, Chang JM, Cho N, Chung SY, Han W, Moon WK. Outcome of breast lesions detected at screening ultrasonography. Eur J Radiol. 2012; 81:3229–3233. PMID: 22591758.

19. Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013; 269:701–712. PMID: 23962417.

20. Chang JM, Koo HR, Moon WK. Radiologist-performed hand-held ultrasound screening at average risk of breast cancer: results from a single health screening center. Acta Radiol. 2015; 56:652–658. PMID: 24951614.

21. Moon HJ, Jung I, Park SJ, Kim MJ, Youk JH, Kim EK. Comparison of cancer yields and diagnostic performance of screening mammography vs. supplemental screening ultrasound in 4394 women with average risk for breast cancer. Ultraschall Med. 2015; 36:255–263. PMID: 24764212.
22. Feig S. Cost-effectiveness of mammography, MRI, and ultrasonography for breast cancer screening. Radiol Clin North Am. 2010; 48:879–891. PMID: 20868891.

23. Irwig L, Houssami N, van Vliet C. New technologies in screening for breast cancer: a systematic review of their accuracy. Br J Cancer. 2004; 90:2118–2122. PMID: 15150556.

24. Moon HJ, Kim EK, Kwak JY, Yoon JH, Kim MJ. Interval growth of probably benign breast lesions on follow-up ultrasound: how can these be managed? Eur Radiol. 2011; 21:908–918. PMID: 21113596.

25. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995; 196:123–134. PMID: 7784555.

26. Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR Am J Roentgenol. 1998; 170:109–114. PMID: 9423610.

27. Kim SJ, Ko EY, Shin JH, Kang SS, Mun SH, Han BK, et al. Application of sonographic BI-RADS to synchronous breast nodules detected in patients with breast cancer. AJR Am J Roentgenol. 2008; 191:653–658. PMID: 18716090.

28. Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dünser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000; 21:325–336. PMID: 11014255.

29. Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer. 2008; 44:539–544. PMID: 18267357.

Figure 1
Flow chart of study population.
US=ultrasound; BI-RADS=Breast Imaging Reporting and Data System; F/U=follow-up.
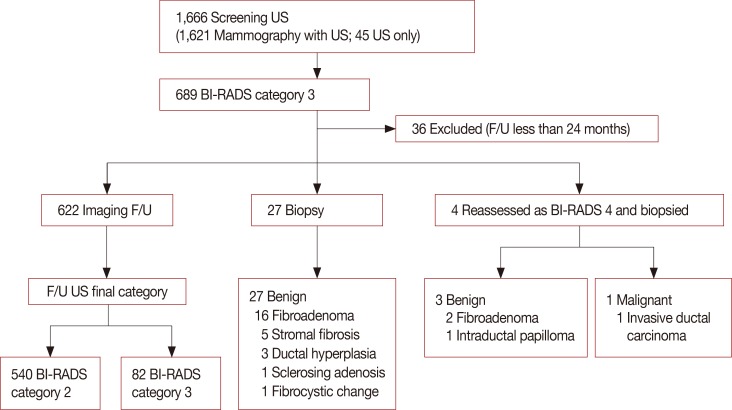
Figure 2
A 52-year-old woman with a mass detected on the screening breast ultrasound (US) in left breast, upper center. (A) An initial US image shows a 1.0 cm oval shape circumscribed isoechoic mass corresponding to Breast Imaging Reporting and Data System (BI-RADS) category 3 on US (arrows). (B) and (C) at the 12 months and 2 years follow-up US, the mass in the left upper center was not changed, and downgraded to BI-RADS category 2.
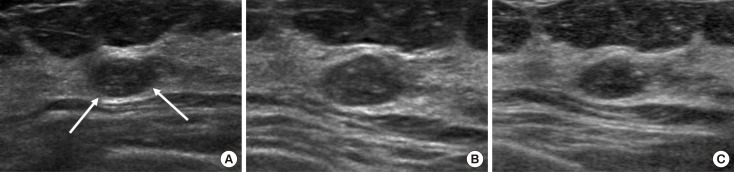
Figure 3
A 40-year-old woman with a nodule detected on the screening breast ultrasound (US) in right breast, upper inner quadrant. (A) An initial US image shows a 0.5 cm oval circumscribed isoechoic mass, corresponding to category 3 (arrows). (B) On the 6-month follow-up US, the surrounding tissue around the previous mass consisted of nodule together, and the nodule showed more district angular margin, more decreased echogenicity and increased size, and was assessed as category 4 (arrows). US-guided core needle biopsy revealed invasive ductal carcinoma, and the patient underwent right partial mastectomy.
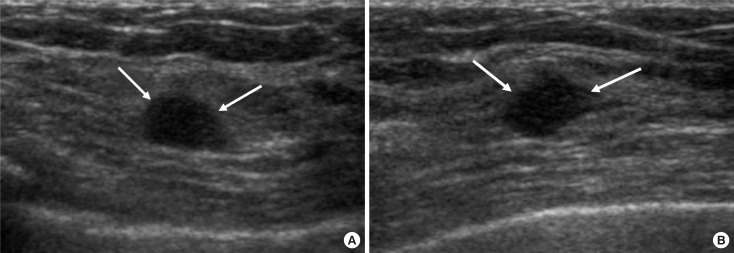
Figure 4
A 45-year-old woman with a mass detected on the screening breast ultrasound (US) in right breast, upper outer quadrant. (A) Initial US image showed a 1.2 cm oval circumscribed isoechoic mass in the right upper outer quadrant, corresponding to category 3 (arrows). (B) On the 12 month follow-up US, the margin of the mass changed into more microlobulated and indistinct, and was assessed as category 4 (arrows). US-guided core needle biopsy was performed and fibroadenoma was confirmed.
Table 1
US findings of BI-RADS category 3 lesions detected at screening US




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download